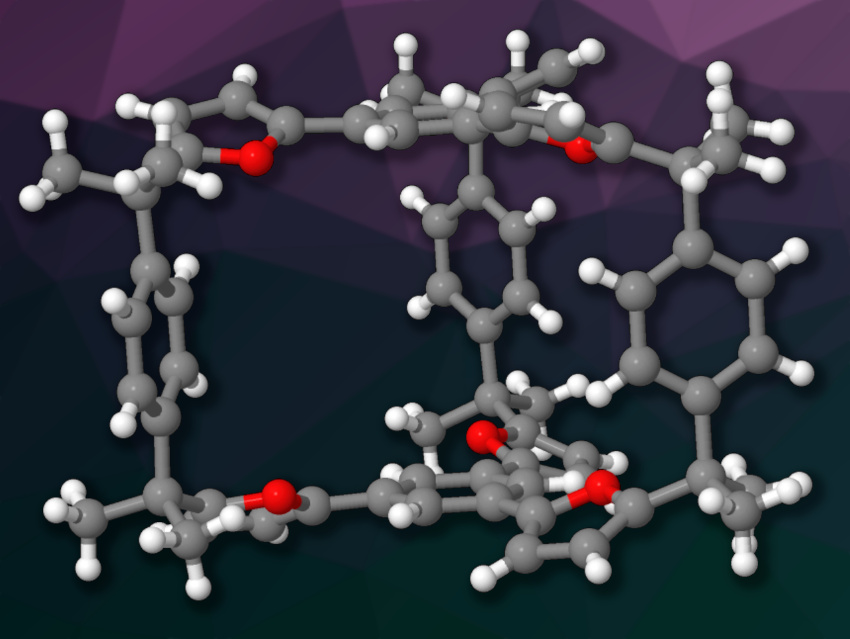
One important research goal in supramolecular chemistry is the construction of host molecules with cavities that can accommodate specific guests. Cage-like molecules can be useful in this context. However, the synthesis of cages from smaller building blocks via irreversibly formed bonds can be challenging due to the formation of unwanted oligomeric byproducts. The use of reversibly formed bonds for the connections can lead to a higher yield of the desired products, but also makes the products less stable.
M. Mustafa Cetin, Kadir Has University, Istanbul, Turkey, Hao Li, Zhejiang University and ZJU-Hangzhou Global Scientific and Technological Innovation Center, Hangzhou, China, and colleagues have synthesized a prism-shaped cage-type molecule (pictured) using a Lewis-acid-catalyzed Friedel–Crafts reaction. This reaction can be considered reversible while the Lewis acid catalyst is present, but the stability of the product is not affected once the catalyst is removed.
The cage was synthesized by condensing 1,3,5-tri(furan-2-yl) benzene and 1,4-di(prop-1-en-2-yl) benzene in a 2:3 ratio via a Friedel–Crafts reaction. The team achieved a yield of ca. 40 %. The product is π-electron rich and consists of two trisfuryl “platforms” connected by three p-xylylene “pillars”. It can accommodate π-electron-poor guests. According to the researchers, the approach could also allow the preparation of target molecules with more complex structures.
- A π-Electron Rich Cage via the Friedel–Crafts Reaction,
Dingsheng Zhu, Bin Sun, Lu Tong, Yating Wu, M. Mustafa Cetin, Hao Li,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c03560