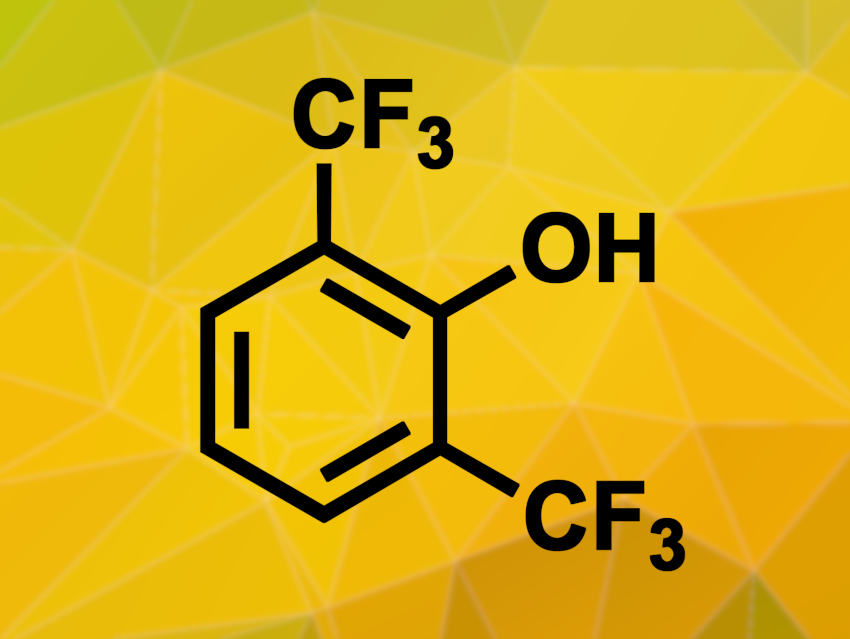
Introducing trifluoromethyl groups into organic molecules can alter their properties in ways that are useful, e.g., in pharmaceutical chemistry. The functionalization of aromatic compounds with multiple trifluoromethyl groups, however, can be challenging and often involves harsh reaction conditions.
Takashi Nishikata, Yamaguchi University, Japan, and colleagues have developed a method for the visible-light-promoted multiple trifluoromethylation of phenol derivatives using commercially available CF3I as the trifluoromethyl source. The team reacted the phenols with two equivalents of CF3I in the presence of Cs2CO3 as a base in dimethylformamide (DMF) as the solvent under 450 nm LED light room temperature.
Under these conditions, doubly trifluoromethylated phenols (parent compound pictured) were obtained. In some cases, the yields could be improved by using a cyanoarene-based photocatalyst. Molecules with multiple phenol units were also suitable substrates, leading to the introduction of four trifluoromethyl groups in one go.
The researchers propose a reaction mechanism that involves a sequential functionalization via single-electron transfers from a photoexcited phenoxide intermediate to CF3I. Overall, the work provides access to multi-CF3-substituted phenols that could be useful, e.g., in drug development or functional materials.
- Continuous activation of phenoxide and CF3I for multiple trifluoromethylations,
Yusei Nakashima, Shinjiro Kusano, Tsukasa Inishi, Yasuyuki Nitta, Takashi Nishikata,
Chem. Commun. 2025.
https://doi.org/10.1039/D4CC06221C