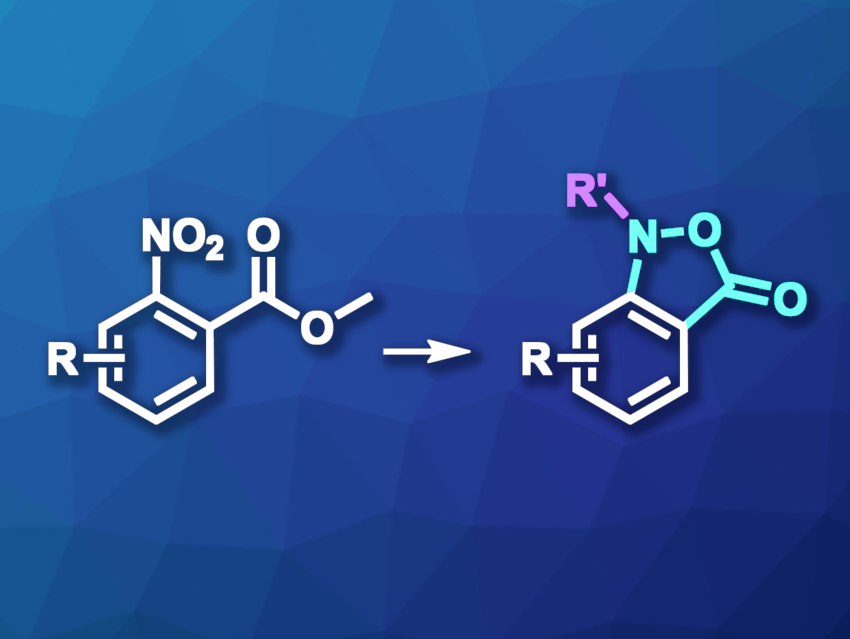
Isoxazolidines are five-membered rings with neighboring nitrogen and oxygen atoms. This substructure, as well as fused benzisoxazoles and benzisoxazolones, are often found in bioactive compounds that are of interest in pharmaceutical chemistry. New and improved methods for their synthesis would, thus, be useful. For example, known methods for the preparation of 2,1-benzisoxazolones can have drawbacks such as low yields or a need for complex starting materials.
David Crich, University of Georgia, Athens, USA, and colleagues have developed a practical method for the synthesis of N-alkyl-1,3-dihydro-2,1-benzisoxazole derivatives (general reaction pictured), as well as the corresponding 2,1-benzisoxazolones, from methyl 2-nitrobenzoates. The nitro groups of the methyl 2-nitrobenzoates were first partially reduced using hydrazine and an Rh/C catalyst to give hydroxylamine derivatives, which then underwent a cyclization and an alkylation to give benzisoxazolone intermediates. For the alkylation step, the researchers used, e.g., benzylic and allylic halides as electrophiles, as well as a primary alkyl iodide.
From the obtained ketone-functionalized intermediates, a reduction step then gave the desired corresponding N-alkyl-1,3-dihydro-2,1-benzisoxazole derivatives in moderate to good yields. For this step, the team used lithium aluminum hydride (LAH) as the reductant and trimethylsilyl chloride (TMSCl) as an additive. Overall, the developed approach provides access to a variety of 2,1-benzisoxazolones and 1,3-dihydro-2,1-benzisoxazoles, which could have applications in drug development.
- Synthesis of N-Alkyl-1,3-dihydro-2,1-benzisoxazoles,
Thomas D. Beckler, David Crich,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c03509