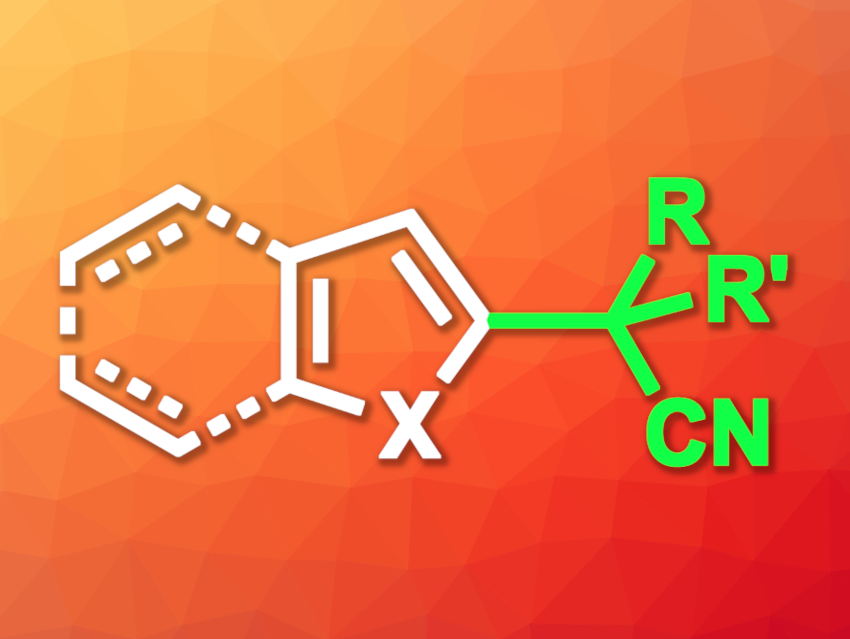
Nitriles with α-aryl or heteroaryl substituents are useful products and intermediates in organic synthesis. Such structures are found in bioactive compounds, and the nitrile groups can also be further transformed into, e.g., esters, amides, carboxylic acids, etc. Sterically hindered derivatives such as α,α-dialkyl-α-aryl nitriles can be challenging to synthesize.
Luis D. Miranda, Universidad Nacional Autónoma de México, Mexico City, and colleagues have developed a method for the synthesis of α,α-dialkyl-α-aryl nitriles via a copper-mediated radical α-heteroarylation. The team used azobis(alkylcarbonitriles) as precursors for α,α-dialkylnitrile radicals and reacted them with different heterocycles in the presence of Cu(OAc)2 as an oxidant in 2,2,2-trichloroethanol (TCE) as the solvent at 110 °C.
Under these conditions, the azobis(alkylcarbonitriles) undergo thermal cleavage, releasing molecular nitrogen and two α,α-dialkylnitrile radicals. One of these radicals can then add to the arene reaction partner, followed by a single-electron oxidation to give a cationic intermediate and deprotonation to give the product.
The desired sterically hindered α,α-dialkyl-α-aryl nitriles were obtained in low to good yields, depending on the reaction partners. The team found that benzofurans and furans were well-suited to the reaction, while pyrroles, indoles, and thiophenes gave lower yields. The reaction is regioselective and tolerates a range of functional groups. Overall, the work is useful for the introduction of quaternary all-carbon centers to heteroarenes.
- A Copper-Mediated Radical α-Heteroarylation of Nitriles with Azobis(alkylcarbonitriles),
Gustavo G. Flores-Bernal, Luis D. Miranda,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.3c03727