Photocatalysis can enable unique transformations using light. However, current photocatalysts are typically homogeneous metal complexes or organic dyes which can be expensive, toxic, and difficult to recycle. Porous organic polymers (POPs) could be useful in the next-generation of metal-free heterogeneous photocatalysts, which are easily recyclable, tunable, and can possess high activities. However, existing methods to make photoactive POPs often use costly monomers and precious-metal catalysts, and the POPs are mainly used in a narrow set of basic photocatalytic transformations.
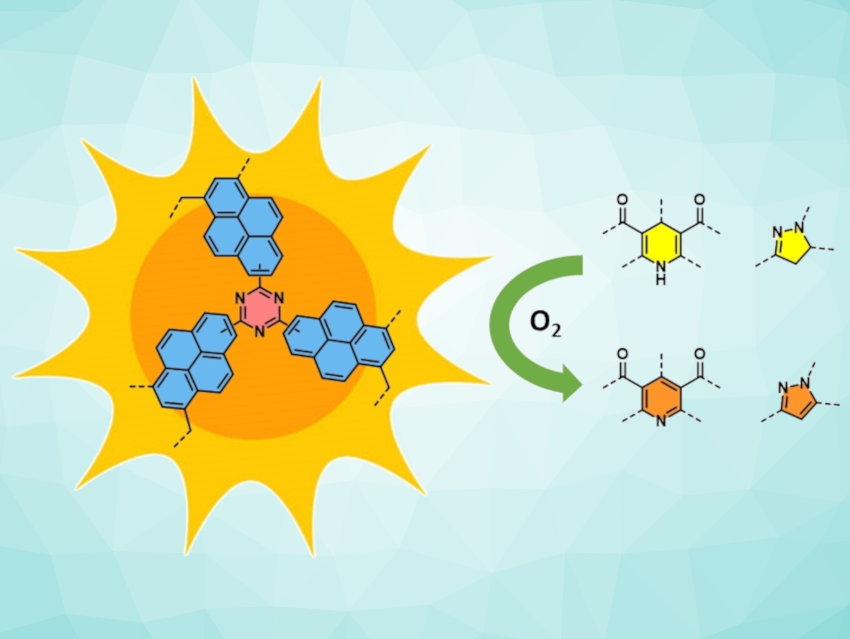
Thomas S. A. Heugebaert, Ghent University, Belgium, and colleagues have synthesized a range of POPs from inexpensive aromatic building blocks through Lewis acid-catalyzed polymerization. The obtained materials, which possess high porosity and excellent photochemical properties, were used as heterogeneous photocatalysts for the aromatization of N-heterocycles. The most active material, CTF-Pyr (pictured) was synthesized by crosslinking pyrene and cyanuric chloride.
CTF-Pyr was used to produce a range of pyridines, dihydroquinoline-5-ones, tetrahydroacridine-1,8-diones, and pyrazoles in good to excellent yields (70–99 %). The transformations took place under mild conditions (under air and at room temperature), highlighting the potential of the developed POPs as a convenient alternative to commonly used homogeneous photocatalysts.
- Development of Porous Organic Polymers as Metal‐Free Photocatalysts for the Aromatization of N‐Heterocycles,
Maarten Debruyne, Nathan Raeymackers, Henk Vrielinck, Sambhu Radhakrishnan, Eric Breynaert, Maxime Delaey, Andreas Laemont, Karen Leus, Jonas Everaert, Hannes Rijckaert, Dirk Poelman, Rino Morent, Nathalie De Geyter, Pascal Van Der Voort, Veronique Van Speybroeck, Christian V. Stevens, Thomas S. A. Heugebaert,
ChemCatChem 2023.
https://doi.org/10.1002/cctc.202301205