Halogen bonding (XB) is a non-covalent interaction between Lewis-acidic halogenated compounds and a Lewis base. It has primarily been used in crystal engineering so far, but the effect is also interesting in the context of organocatalysis, for example, in halide abstraction reactions. However, most halogen-bonding organocatalysts are discarded after one run of the respective reaction.
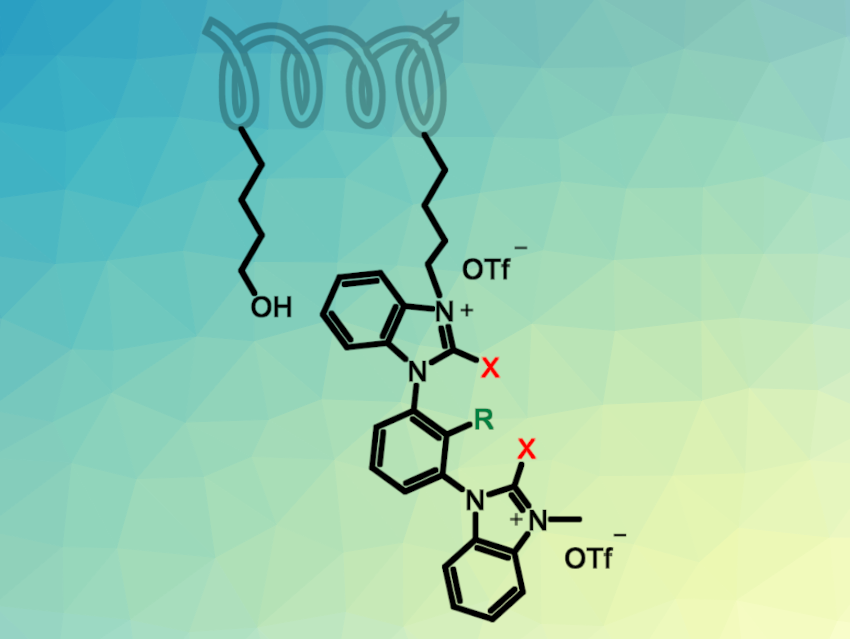
To make the use of halogen-bonding organocatalysts more sustainable, Stefan M. Huber, Ruhr-University Bochum, Germany, and colleagues have immobilized bis(benzimidazolium)-based XB units on a polystyrene resin (pictured above). The team started from a commercially available polystyrene functionalized with hydroxybutyl sidechains. They first converted the hydroxy groups into better leaving groups using triflic anhydride. The hydrogen-bond donor groups were then introduced via nucleophilic substitution reactions with bis(benzimidazole)benzene-based units. Finally, an N-methylation led to potent dicationic XB donor substituents (see above, R = H, CF3; X = halogen).
The team obtained an insoluble catalyst that was tested in a simple halide-abstraction benchmark reaction between 1-chloroisochroman and a ketenesilyl acetal (pictured below).
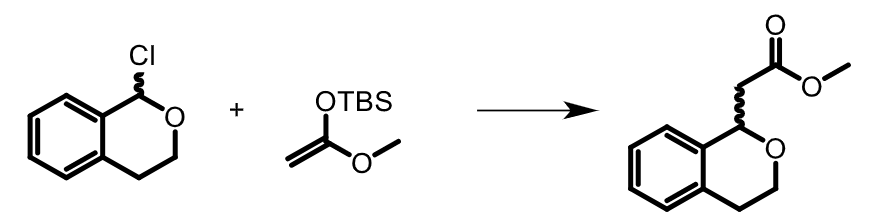
Almost quantitative yields were observed after a reaction time of 6 h with a catalyst load of 10 mol%. The resin was reused in three consecutive runs with only small changes in catalytic activity. According to the researchers, this represents the first example of a polymer-bound recyclable XB organocatalyst. Further optimization of these XB donors could allow, e.g. the extension of their use for other types of reactions.
- Polymer‐Bound Halogen Bonding Organocatalysis,
Raffaella Papagna, Dana Kutzinski, Stefan Matthias Huber,
Eur. J. Org. Chem. 2022.
https://doi.org/10.1002/ejoc.202200852