Polystyrene is a widely used polymer that is essentially not recyclable when mixed with other materials and is not biodegradable. Ulrich Schwaneberg, Jun Okuda, RWTH Aachen University, Germany, and colleagues have developed a biohybrid catalyst that oxidizes polystyrene microparticles to make them easier to degrade. The catalyst consists of a specially constructed “anchor peptide” that adheres to polystyrene surfaces and a catalytically active cobalt cofactor.
Polystyrene Recycling Can Be Difficult
Polystyrene—alone or in combination with other polymers—has many applications, from yogurt containers to instrument cases. In foam form, mainly known under the trademarked name Styrofoam, it is, for example, used for insulation and packaging.
A big disadvantage of polystyrene is its poor biodegradability, which leads to environmental pollution. When clean and not mixed with other materials, polystyrene is recyclable, but not when it is contaminated or combined with other materials. In municipal recycling programs, mixed polystyrene plastic waste and degradation products, such as polystyrene nano- and microparticles, are difficult to process. The problem lies in the fact that polystyrene is water-repellent and nonpolar, and thus, cannot react with common polar reactants.
Introducing Polar Groups Using an Anchored Catalyst
For a simple, economical, and energy-efficient process to break down mixed polystyrene waste, the polystyrene must first be equipped with polar functional groups. Groups such as hydroxyl and carbonyl groups can be introduced by selective C−H bond functionalization.
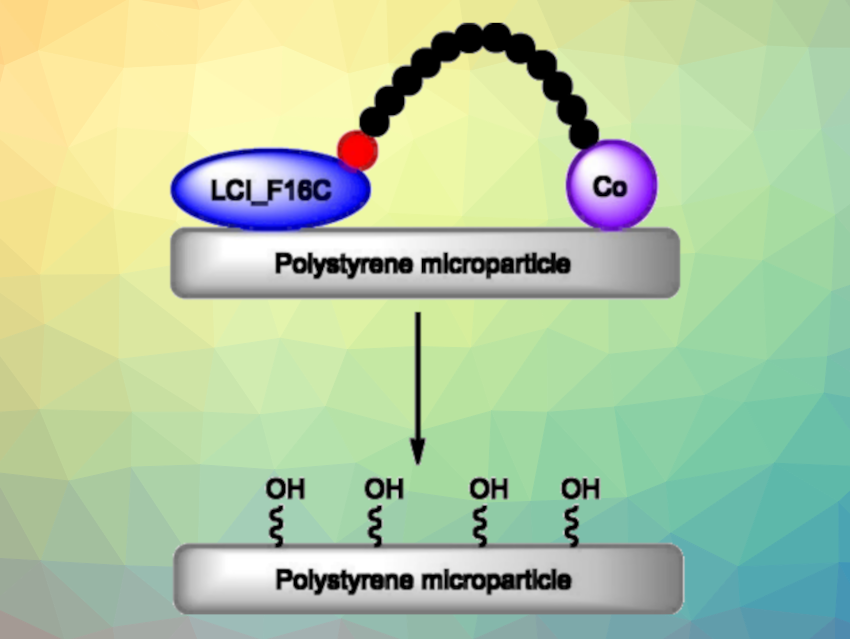
The researchers developed a biohybrid catalyst to carry out this step. The catalyst is based on “anchor peptides” bound to a cobalt cofactor. Anchor peptides are short peptide chains that can interact with surfaces through different non-covalent interactions.
Biohybrid Catalyst Boots Hydroxylation
The team developed an engineered anchor peptide (LCI_F16C, LCI = “Liquid Chromatography Peak I”) that binds to the surface of polystyrene. One gram of this peptide is enough to coat a surface of up to 654 m2 with a monolayer within minutes by either spraying or dipping.
A catalytically active cobalt complex is attached to the anchor peptide via a short linking piece. The cobalt atom is surrounded by the macrocyclic ligand TACD (1,4,7,10-tetraazacyclododecane). The catalyst accelerates the oxidation of the C–H bonds in polystyrene to form polar OH groups (pictured) in a reaction with commercially available oxone (potassium peroxymonosulfate as an oxidant. The oxidation using an optimized biohybrid catalyst resulted in a 15 times higher functionalization level than using the corresponding free cobalt cofactors.
A Scalable and Energy-Efficient Process
The binding of the anchor peptides is material-specific, so in this case they immobilize the catalytically active cobalt near the polystyrene surface, which accelerates the reaction. This simple, inexpensive, and energy-efficient process is scalable through dipping and spray applications and is suitable for use on an industrial scale.
Through the use of conjugated chemical catalysts, this hybrid catalyst concept using material-specific binding by anchor peptides could also allow the targeted breakdown of other hydrophobic polymers, such as polypropylene and polyethylene, that cannot be economically broken down by enzymes.
- Engineered Anchor Peptide LCI with a Cobalt Cofactor Enhances Oxidation Efficiency of Polystyrene Microparticles,
Dong Wang, Aaron A. Ingram, Julian Luka, Maochao Mao, Leon Ahrens, Marian Bienstein, Thomas P. Spaniol, Ulrich Schwaneberg, Jun Okuda,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202317419