Modern medicinal chemists aim to create complex molecules with a high proportion of sp3 hybridized carbons because these three-dimensional structures can better interact with complicated biological targets. This focus increases the ability of molecules to specifically bind to their intended targets and improves their physicochemical properties, such as solubility and stability, which are critical for effective drug development. Historically, synthetic methods have produced flat, high sp2 structures using techniques such as electrophilic aromatic substitution followed by Pd-based cross-coupling.
Yu Kawamata and Hans Renata, Rice University, Houston, TX, USA, Phil S. Baran, Scripps Research, La Jolla, CA, USA, and colleagues have developed an approach for the rapid, modular, enantioselective construction of piperidine framework by combining scalable biocatalytic methods with radical cross-coupling. This new approach to synthesizing complex, three-dimensional molecules streamlines the production of high-value enantiopure piperidines and simplifying the synthesis of medicinally important molecules.
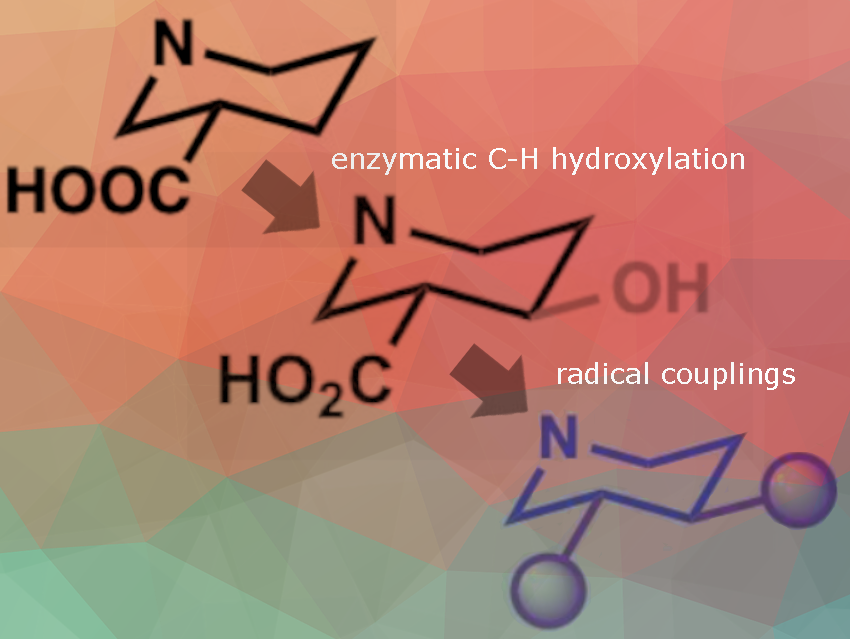
First, the team used scalable enzymatic C–H oxidation to introduce hydroxyl groups into inexpensive 2- and 3-carboxylated piperidines (pictured). The researchers used trans-4-proline hydroxylase (trans-P4H) and an engineered proline-4-hydroxylase (P4H810). There were no examples of enzymes that catalyze the hydroxylation of 3-carboxylated piperidine. The team found ectoine 5-hydroxylase from Sphingopyxis alaskensis (SaEctD).
The hydroxylated intermediates then undergo radical cross-coupling reactions, enabling the modular and enantioselective assembly of complex, three-dimensional piperidine derivatives. The radical cross-coupling methods used involved Ni-electrocatalytic decarboxylative cross-coupling, which facilitated the chemoselective coupling of hydroxypiperidines with aryl iodides. For example, N-Boc-3-hydroxy-2-phenylpiperidine was synthesized directly in a more straightforward and diastereoselective manner. Similarly, the synthesis of the natural product swainsonine was simplified through Ni-electrocatalytic decarboxylative alkenylation, bypassing several lengthy and labor-intensive steps while avoiding the need for expensive transition metals and cryogenic conditions.
- Biocatalytic C–H Oxidation Meets Radical Cross-Coupling: Simplifying Complex Piperidine Synthesis, Jiayan He, Kenta Yokoi, Breanna Wixted, Benxiang Zhang, Yu Kawamata, Hans Renata, Phil Baran,
ChemRxiv 2024.
https://doi.org/10.26434/chemrxiv-2024-9ks60
This content is a preprint and has not undergone peer review at the time of posting.