Pillar[n]arenes are macrocycles composed of arene units linked via methylene groups. They have a belt-like structure and can be useful in host-guest chemistry. There are various examples of functionalized pillararenes tailored to different applications, usually at the top and bottom edges of the “belt” or at the methylene bridges. Introducing heteroatoms directly into the belt can also lead to interesting properties and new possible applications of pillararenes.
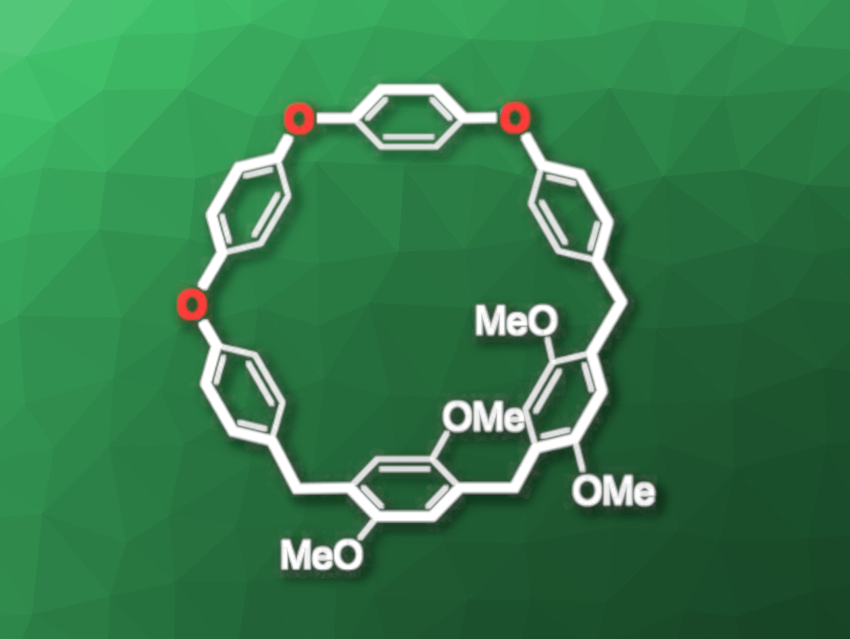
Weijian Xue, Qiqihar University, China, and colleagues have synthesized pillararenes with partial oxygen functionalization in the belt (example pictured). The team prepared an oxygen-substituted tetramer using a Friedel–Crafts reaction and an Ullmann condensation reaction. The ends of the tetramer were converted to benzyl alcohol groups and then it was reacted with a tetramethyloxyl-functionalized dimer to obtain a pillar[6]arene with three oxygen atoms in the belt (pictured). The researchers also reacted the tetramer with 1,4-dimethoxybenzene and obtained a large pillar[10]arene with six oxygen atoms in the belt in a yield of 9 %.
The oxygen-functionalized pillar[6]arene was characterized using X-ray diffraction, and the team found that the structure has a cavity when viewed from the top, but different from “traditional” pillararenes, the bridging atoms are not all in the same plane and it has a figure-eight-like structure in the side view. The oxygen-functionalized pillar[10]arene has a rectangular cavity and a structure in which the the bridging atoms are closer to co-planar when viewed from the side. According to the team, these novel structures could be useful, e.g., in molecular recognition.
- Step Cyclization to Give Part Belt Oxygen-Functionalized Pillar[6,10]arenes,
Weijian Xue, Yuhang Liu, Huiqian Li, Xiangyu Ling, Bing Zhao, Yanbing Yin,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c04607