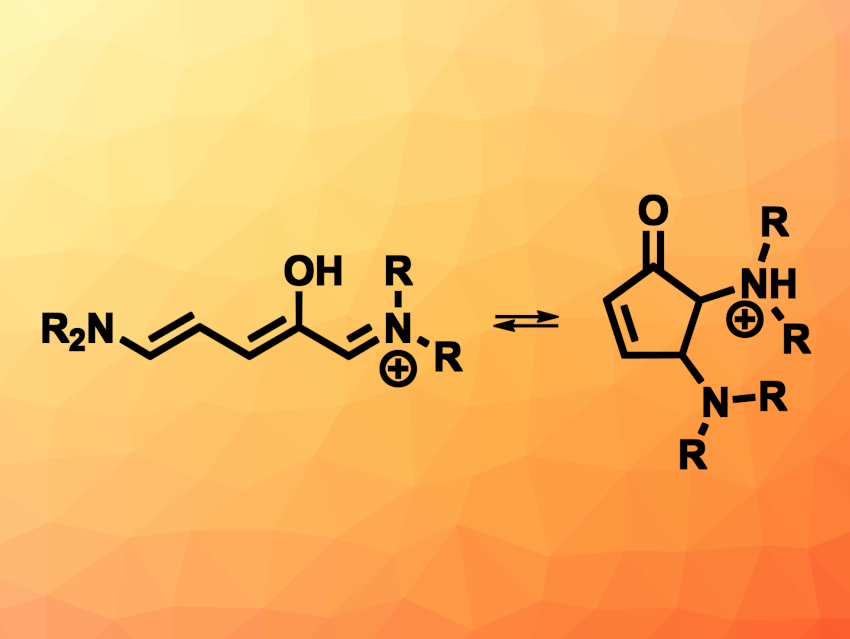
Molecular photoswitches can be useful to create functional, stimuli-responsive materials. In particular for medical or pharmaceutical applications, photoswitches that respond to non-harmful visible or near-infrared light are interesting. In addition, photoswitches for use in biological systems need to function well in aqueous solutions. Many common photoswitches do not fulfill these requirements because they often need UV light for switching and/or are not well-soluble in water. Donor–acceptor Stenhouse salts, which are the products of a furan ring-opening reaction upon the condensation of furfurals and amines, can serve as photoswitches via a reversible ring-closing mechanism. However, they have often been limited to use in nonpolar solvents.
Julie A. Peterson, Bowling Green State University, Bowling Green, OH, USA, and colleagues have developed a series of aniline-based Stenhouse salt photoswitches with electron-donating and electron-withdrawing groups (general structure pictured). These photoswitches can function in protic solvents such as deionized water or methanol. The team synthesized the photswitches from furfural and a series of aniline derivatives with substituents such as methyl, fluorine, chlorine, bromine or cyano groups.
The researchers found that the resulting photoswitches absorb green light and show rapid and reversible photochromism in polar protic solvents, changing from a visible-light absorbing isomer to a visible-light transparent isomer upon irradiation and ring-closing. This switch happens in common protic solvents, as well as in hydrogels. The equilibrium between the open and closed forms can be tuned by changing the substituents at the aniline unit. The developed aniline-based Stenhouse salt photoswitches could serve as useful alternatives to other commonly used organic photoswitches.
- Stenhouse Salts: Visible Light Photoswitches for Protic Environments,
Derek Puthoff, Hrishikesh Kuttiyil, Julie A. Peterson,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c13085



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)