Organosulfur compounds are interesting research targets. Sulfur-containing molecules are often found, for example, among natural products and pharmaceutically active compounds and in functional materials. Sulfur-functionalized ketones, for example, can have useful biological activities and serve as intermediates in organic synthesis. β-Keto thiosulfones can often be prepared under transition-metal-catalyzed conditions. Developing alternative metal-free catalytic methods would be useful.
Ping Song, Shun-Yi Wang, Soochow University, China, and colleagues have developed a metal-free, visible-light promoted radical reaction of α-bromoacetophenones with thiosulfonates or selenosulfonates to give β-keto thiosulfones or β-keto selenosulfones, respectively (pictured). The reaction constructs two different C–S bonds or one C–S bond and one C–Se bond simultaneously under mild conditions. The team used 9-mesityl-10-methylacridinium perchlorate (Mes–Acr+–Me ClO4–) as the photocatalyst, K2CO3 as a base, and ethanol as the solvent. The reaction was performed at room temperature under purple light irradiation and an N2 atmosphere.
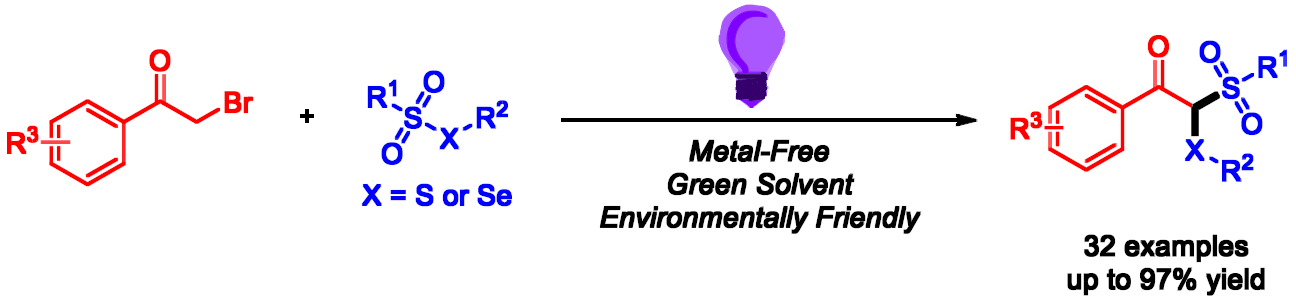
This approach shows a wide substrate scope and good atom economy, and it uses ethanol as a green solvent. The desired products were obtained in moderate to high yields. The team performed a gram-scale reaction and obtained a yield of 74 %.
- Photoinduced Reaction of α‐Bromoacetophenone with Thiosulfonate: Synthesis of β‐Keto Thiosulfone,
Wei‐Chen Zhu, Yi‐Fan Jiang, Xin‐Yu Liu, Shi‐Yin Tian, Weidong Rao, Shu‐Su Shen, Ping Song, Daopeng Sheng, Shun‐Yi Wang,
Eur. J. Org. Chem. 2023.
https://doi.org/10.1002/ejoc.202300379




Bonjour,
Je suis intéressé par votre production de ces molécules organique, j’ai un diplôme d’ingénieur chimiste.