Carboranes are clusters composed of boron, carbon, and hydrogen atoms. They have applications, e.g., in coordination chemistry, catalysis, and supramolecular chemistry. New methods for the synthesis of functionalized carboranes are interesting research targets. It can, however, be challenging to perform such reactions in a selective manner due to the presence of several B–H bonds in similar chemical environments.
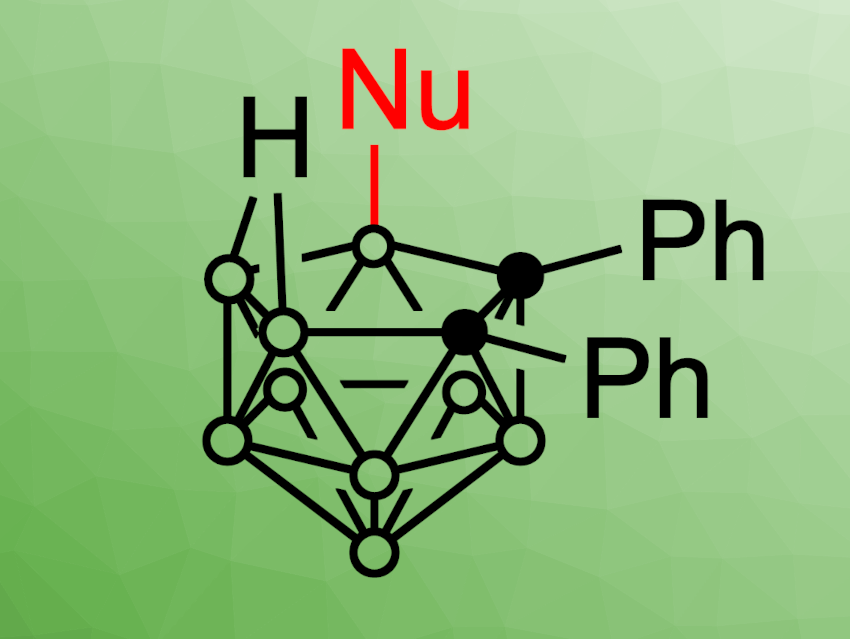
Deshuang Tu, Hong Yan, Nanjing University, China, Xingwei Guo, Tsinghua University, Beijing, China, Jordi Poater, Universitat de Barcelona and ICREA, Barcelona, Spain, Miquel Solà, Universitat de Girona, Spain, and colleagues have developed a method for the photoinduced selective B–H functionalization of open-cage nido-carboranes (example product structure pictured). The team used 9-mesityl-10-methylacridinium perchlorate as a photocatalyst, which can take part in a cage···π interaction with a nido-carborane cage. This noncovalent bond promotes a charge transfer from the cage to the photocatalyst.
Under irradiation with green light, a single electron transfer (SET) process gives a nido-carborane cage radical. In the presence of O2 in air, this radical can undergo a hydrogen atom transfer (HAT). This results in an electrophilic carborane cage that can react with different nucleophiles. Under these conditions, a wide range of functionalized products was obtained in moderate to excellent yields and with high regioselectivity at the B(9) site of the nido-carborane cage.
- Photoinduced Selective B–H Activation of nido-Carboranes,
Shengwen Xu, Hongjian Zhang, Jingkai Xu, Weiqun Suo, Chang-Sheng Lu, Deshuang Tu, Xingwei Guo, Jordi Poater, Miquel Solà, Hong Yan,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c00550