Oxazole derivatives are often found in pharmaceutically active compounds. New methods for their synthesis are, thus, useful—for example, in drug development. Ideally, such methods require few steps, are atom-economic, have a broad substrate scope, and can be scaled up successfully. Photoinduced methods can be interesting in this context.
Debabrata Maiti, Indian Institute of Technology Bombay, Mumbai, India, and colleagues have developed a protocol for the synthesis of pharmaceutically useful oxazole derivatives via a photoinduced [3+2] cycloaddition of carbenes and nitriles (general product structure pictured above). The team started from α-carbonyl diazo compounds, which were reacted with nitriles in dichloroethane (DCE) under blue LED light over 24 h.
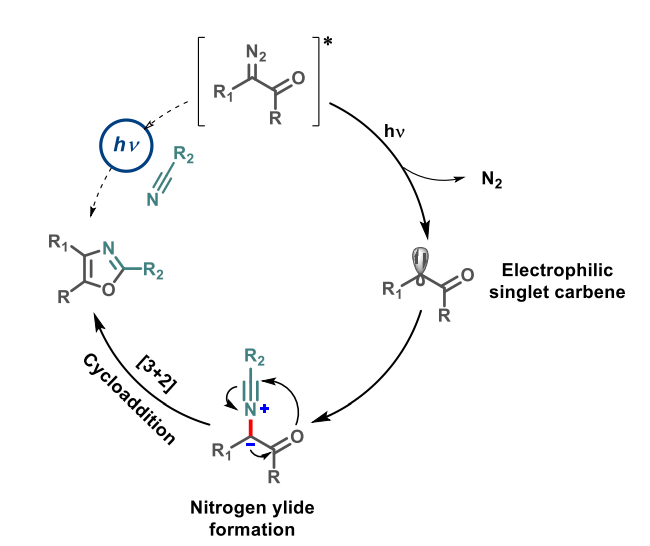
The desired oxazoles were obtained in moderate to high yields, with a wide spectrum of products. The reaction does not require metal catalysts and can also be used in a flow chemistry approach. The team proposes a mechanism (pictured above) that involves the formation of a carbene from the α-carbonyl diazo compound under light irradiation. The carbene can then be trapped by the nitrile to form a nitrogen ylide intermediate. Finally, an intramolecular [3+2] cycloaddition gives the desired oxazole product.
- Photoinduced [3+2] Cycloaddition of Carbenes and Nitriles: A Versatile Approach to Oxazole Synthesis,
Argha Saha, Chiranjit Sen, Srimanta Guin, Chandan Das, Debajit Maiti, Subhabrata Sen, Debabrata Maiti,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202308916
![Photoinduced [3+2] Cycloaddition of Carbenes and Nitriles](https://www.chemistryviews.org/wp-content/uploads/2023/10/cycloadditionofcarbenesandnitriles_2023.png)



