Curved aromatic compounds are interesting research targets due to their structural and electronic properties, which can differ from typical planar aromatic compounds. Photochromism is the reversible transformation of a compound between two forms upon light irradiation. Efficient photochromic materials require efficient light absorption and high photoreactivity. A curved structure could affect these properties.
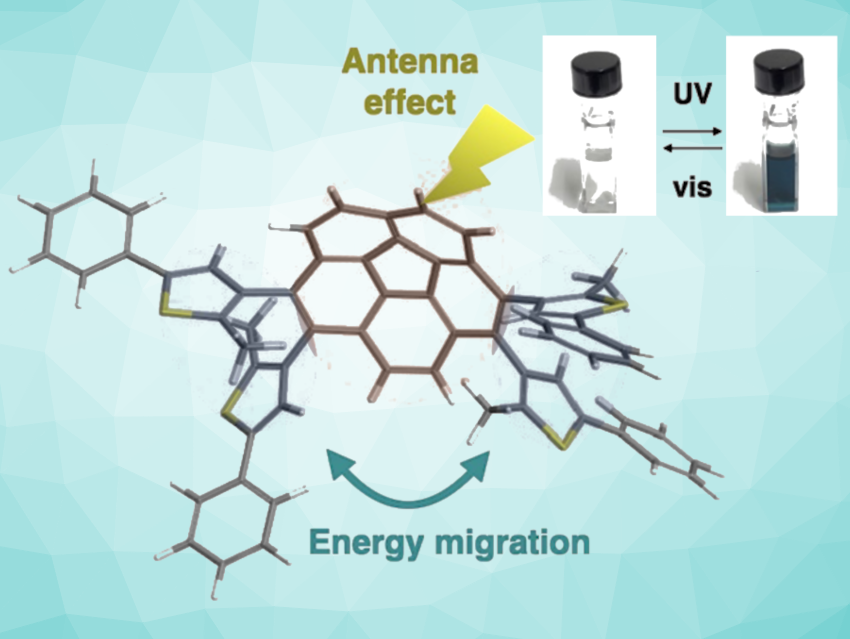
Mihoko Yamada, Tsuyoshi Kawai, and colleagues, Nara Institute of Science and Technology, Ikoma, Japan, have synthesized photochromic tetrathienyl corannulene compounds, 1,2,7,8-tetrakis(2-methyl-5-phenylthiophen-3-yl)corannulene and 1,2,7,8-tetrakis(2,4-dimethyl-5-phenylthiophen-3-yl)corannulene. The compounds feature photochromic terarylenes bound to a central, curved, aromatic corannulene.
The first compound, in particular, exhibits highly sensitive photochromism with efficient light absorption and high photoreactivity. Under light irradiation with either UV or visible light, a C–C bond in the compound is reversibly formed or broken in a photocyclization/photocycloreversion reaction. The conversion ratio between the two forms reached 0.90.
The team explains these promising properties by the antenna effect of the large aromatic corannulene unit, as well as the predominance of the photoreactive atropisomers and amplified energy migration. The distance between two reactive carbons is shortened by the effects of the curved skeleton of corannulene. According to the researchers, taking advantage of curved aromatic skeletons could provide a new approach to controlling photoreactivity via the distortion of molecules.
- Tetrathienyl corannulene compounds with highly sensitive photochromism,
Mihoko Yamada, Tomoya Sawazaki, Mae Fujita, Fumio Asanoma, Yoshiko Nishikawa, Tsuyoshi Kawai,
Chem. Eur. J. 2022.
https://doi.org/10.1002/chem.202201286
![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)

