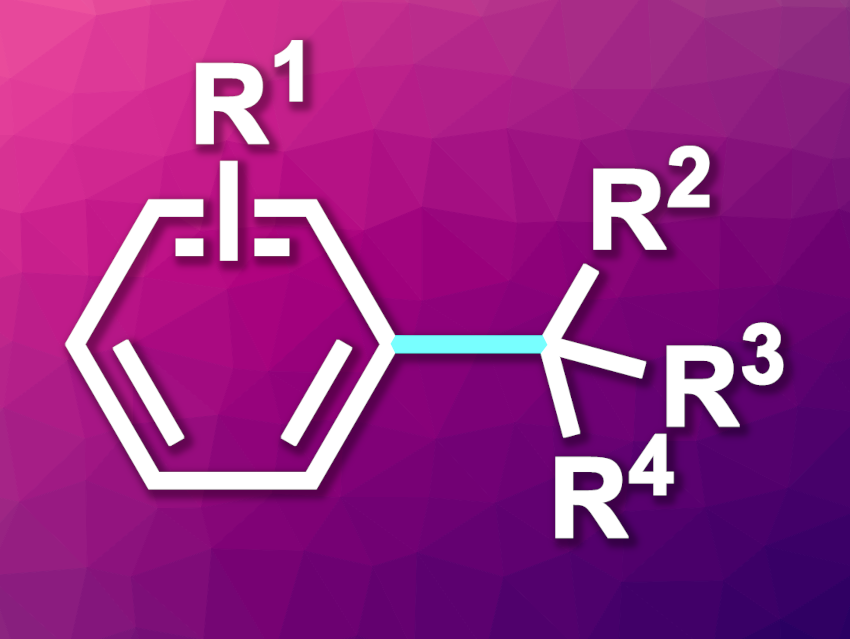
The direct and selective coupling of arenes and aliphatic hydrocarbons, including alkanes and cycloalkanes, could be a useful strategy for C(sp2)–C(sp3) bond formation. This method offers advantages in terms of both atom- and step-economy, while also benefiting from abundant starting materials. However, achieving good reactivity as well as chemo- and site-selectivity can be challenging.
Yu-Mei Lin, Lei Gong, Xiamen University, Fujian, China, and colleagues have developed a photochemical system that uses catalytic amounts of iron(III) halides (FeCl3 or FeBr3) as multifunctional reagents and air as a green oxidant to address this synthetic problem. Under mild conditions, the reaction between a strong C(sp2)–H bond and a robust C(sp3)–H bond has been achieved, giving a broad range of cross-coupling products in high yields and with useful chemo- and site-selectivities.
The iron halide acts as a multifunctional reagent that responds to visible light, initiates the formation of carbon-centered radicals, induces their single-electron oxidation to carbocations, and participates in a subsequent Friedel-Crafts-type process. The gradual release of radical species and carbocation intermediates appears to be critical for achieving the desired reactivity and selectivity. The developed environmentally friendly, cost-efficient approach offers access to various building blocks from abundant hydrocarbon feedstocks, and it shows the potential of iron halides in sustainable synthesis.
- Visible light-triggered selective C(sp2)-H/C(sp3)-H coupling of benzenes with aliphatic hydrocarbons,
Qian-Yu Li, Shiyan Cheng, Ziqi Ye, Tao Huang, Fuxing Yang, Yu-Mei Lin, Lei Gong,
Nat. Commun. 2023.
https://doi.org/10.1038/s41467-023-42191-9