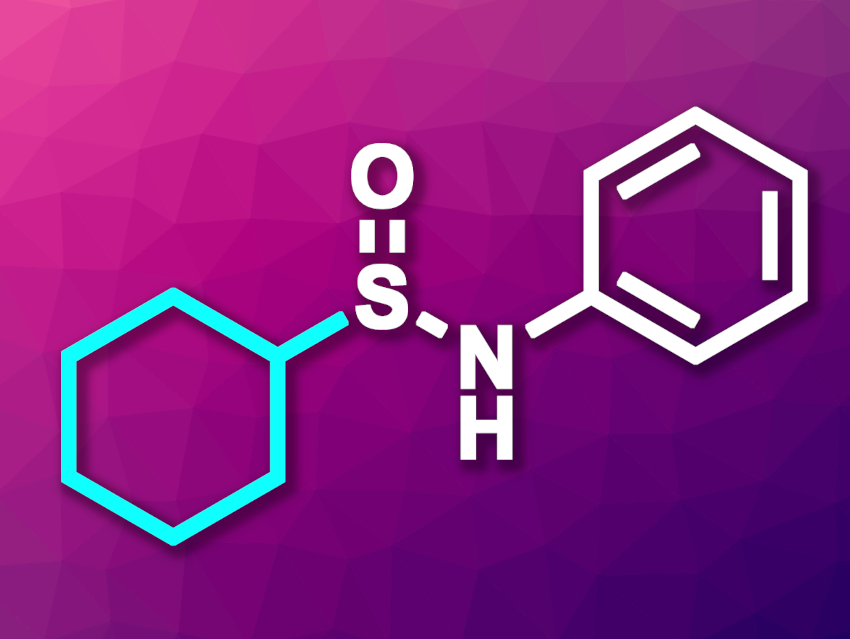
Sulfinamides (R−S(=O)−NR2) are used, e.g., in medicinal chemistry or organic synthesis. They can serve as synthetic precursors for a wide variety of sulfur-containing organic compounds and also have uses as ligands or chiral auxiliaries. Existing methods or the synthesis of sulfinamides can have drawbacks such as low atom economy, a need for unstable reagents, or harsh reaction conditions.
Yi Wei, Xiao-Qiang Hu, South-Central Minzu University, Wuhan, China, and colleagues have developed a method for the iron-catalyzed photochemical sulfinamidation of hydrocarbons with N-sulfinylamines to give sulfinamides (example product pictured). The team reacted a range of N-sulfinylamines with different aliphatic hydrocarbons using FeCl3 as a catalyst and acetonitrile as a solvent under irradiation with LED lights (390 nm) and an N2 atmosphere. The reactions were performed at room temperature.
Under these conditions, the desired sulfinamides were obtained in moderate to high yields. The team also performed the reaction on a gram scale, obtaining a yield of 67 %. The approach was also suitable for the late-stage functionalization of drug-like molecules, and the work could have applications in drug discovery.
- Iron-Catalyzed Photochemical Synthesis of Sulfinamides from Aliphatic Hydrocarbons and Sulfinylamines,
Guang-Da Xia, Run Li, Long Zhang, Yi Wei, Xiao-Qiang Hu,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c00612