Understanding the behavior of uranium is important for nuclear fuel processing where interactions with peroxides play a significant role, especially in processes such as mining, milling, and separation processes such as CARBEX (separating uranium and plutonium from spent fuel with carboxylic acid-based extractants to recover actinides and minimize waste). Radiolysis of water during uranium processing generates reactive oxygen species (ROS) and free radicals that can degrade uranium processing extractants and reduce their effectiveness for uranium and other actinides. To improve processing efficiency, it is important to better understand these reactions.
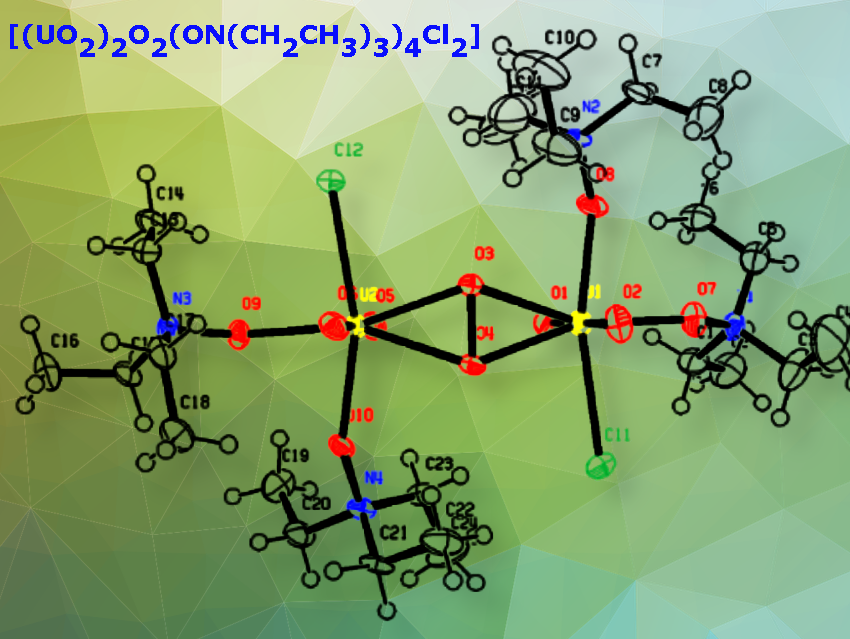
Brett Lottes and Korey P. Carter, University of Iowa, Iowa City, IA, USA, have synthesized a new uranyl peroxide dimer, µ-peroxido-bis[di-triethylamine N-oxide-dichloro-dioxouranium] complex with the formula [(UO2)2O2(ON(CH2CH3)3)4Cl2] (pictured). It was formed photochemically from a reaction involving uranyl chloride, triethylamine, and H2O2. H2O2 is photolytically broken down into •OH radicals and other free radicals. The •OH radicals react in situ with triethylamine to form triethylamine N-oxide (TEAO). These ligands subsequently bind to the UO22+ cations in a monodentate fashion via their N-oxide functional groups. H-atom abstraction at the N+–H bond during irradiation creates these N-oxide groups.
Single crystal X-ray diffraction (SCXRD) showed that TEAO forms strong H-bonds with UO22+ and peroxide moieties. and unique pentagonal bipyramidal geometries of the UO22+ cations. Raman spectroscopy confirmed changes in stretching modes, particularly the C–N stretches, which are red-shifted after oxidation. This indicates that Raman spectroscopy would be able to track amine degradation during the solvent extraction steps of the CARBEX or other spent nuclear fuel separations processes.
Future work of the researchers will include to track the transformation of triethylamine to its N-oxide form and investigate the behavior of TEAO with other fission metals to understand its impact on extractant selectivity in the CARBEX process.
- Photochemical Synthesis and Characterization of a Uranyl Peroxide Triethylamine N-Oxide Dimer,
Brett Lottes, Korey P. Carter,
Eur. J. Inorg. Chem. 2024
https://doi.org/10.1002/ejic.202400300