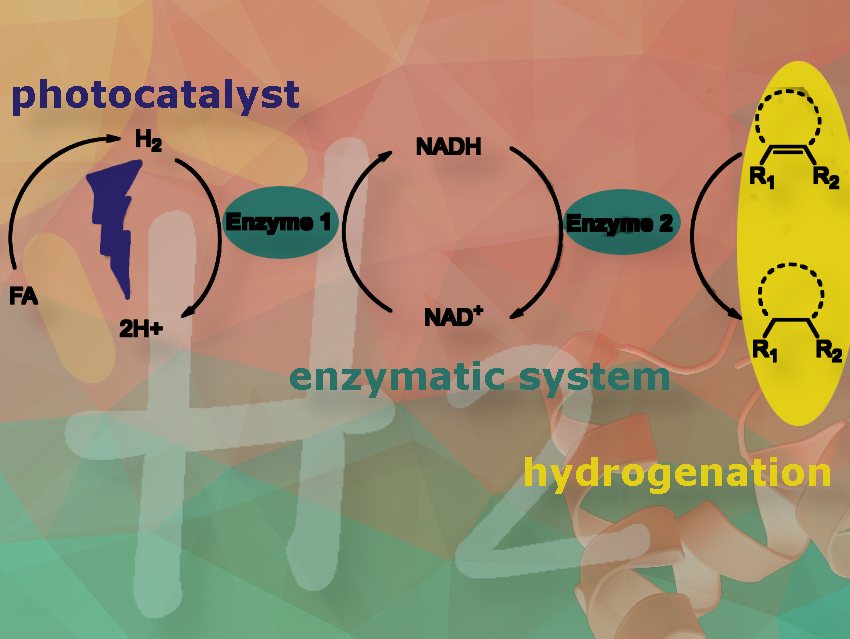
Yong Peng, Leibniz Institute for Catalysis, Rostock, Germany, and colleagues have developed a system that uses light-triggered hydrogen release and enzymatic hydrogenation for controlled chemical synthesis under mild conditions.
Can you say more about the novel concept you introduced in your latest research?
In our latest work submitted to ChemSusChem, we introduced a novel concept that combines photocatalytically triggered hydrogen release from formic acid, a promising liquid organic hydrogen carrier (LOHC), with enzymatic hydrogenation to produce industrially valuable chemicals under mild conditions. The key highlight of our results is the ability to control the release of H2 from LOHCs with light.
The system achieves an exceptionally high H2 releasing activity that can be switched on or off controlled by light, effectively driving the enzymatic hydrogenation reactions.
What interests you about this?
Conventional hydrogenation reactions are essential for producing everyday commodities but often rely on fossil fuels and necessitate harsh conditions. In contrast, enzymatic reactions can occur under much milder conditions.
Our goal was to develop a system that uses light to trigger the on-site release of hydrogen from LOHCs, integrating it with enzymatic processes to create a cleaner and more sustainable hydrogenation method, thus, to contribute to the reduction of carbon emissions.
What else is new and cool about your system?
Enzymatic reactions and photocatalytic H2 evolution are distinct research areas, and it’s exciting for us, as a team, to learn from each other on this interdisciplinary topic and tackle challenges through collaboration. We believe that our approach could inspire further research and development in the fields of green chemistry and renewable energy.
What is the main significance of your results?
The most significant aspect of our results is the achievement of a very high H2 release rate driven solely by light irradiation, which has been shown to be sufficient to fuel the enzymatic hydrogenation reactions. Moreover, we believe that the photocatalytic H2 release system holds great promise for practical applications involving gaseous H2, such as H2 batteries.
What is the longer-term vision for your research?
With advancements in green hydrogen and LOHC technologies, we can foresee widespread adoption of on-site H2 release systems, e.g., in areas such as automotive, pharmaceuticals, and renewable energy. We envision that light-triggered hydrogen release systems will become a focal point for further research, particularly in optimizing these systems for different LOHCs and exploring other enzymatic processes that could benefit from on-site hydrogen generation.
What part of your work was the most challenging?
The biggest challenge was coupling the H2 release with the enzymatic reactions.
As a chemist, I wasn’t very familiar with enzymes and their tolerance to different conditions. For example, we initially struggled to get a good conversion in the enzymatic reaction without understanding why. After several control experiments and discussions within the team, we discovered that high concentrations of CO2 were the reason for the low activity of the enzymatic reaction.
Despite some difficulties, I truly enjoyed this interdisciplinary collaboration and would like to express my sincere gratitude to all my co-authors for their invaluable contributions to this work. As I mentioned, this project is interdisciplinary, and achieving our goals would not have been possible without collaboration and teamwork.
Thank you very much for these insights into your work.
The paper they talked about:
- State-of-the-Art Light-Driven Hydrogen Generation from Formic Acid and Utilization in Enzymatic Hydrogenations,
Matthias Beller, Yong Peng, Thaleia Sakoleva, Nils Rockstroh, Stephan Bartling, Pierre Schoenmakers, Guiyeoul Lim, Duo Wei, Thomas Bayer, Mark Dörr, Dominique Böttcher, Lars Lauterbach, Henrik Junge, Uwe T. Bornscheuer,
ChemSusChem 2024.
https://doi.org/10.1002/cssc.202401811

Yong Peng is currently a Marie Curie postdoctoral fellow working with Professor Matthias Beller at the Leibniz Institute for Catalysis in Rostock, Germany.
His research focuses on the photocatalytic H2 evolution and C–H activation and functionalization.