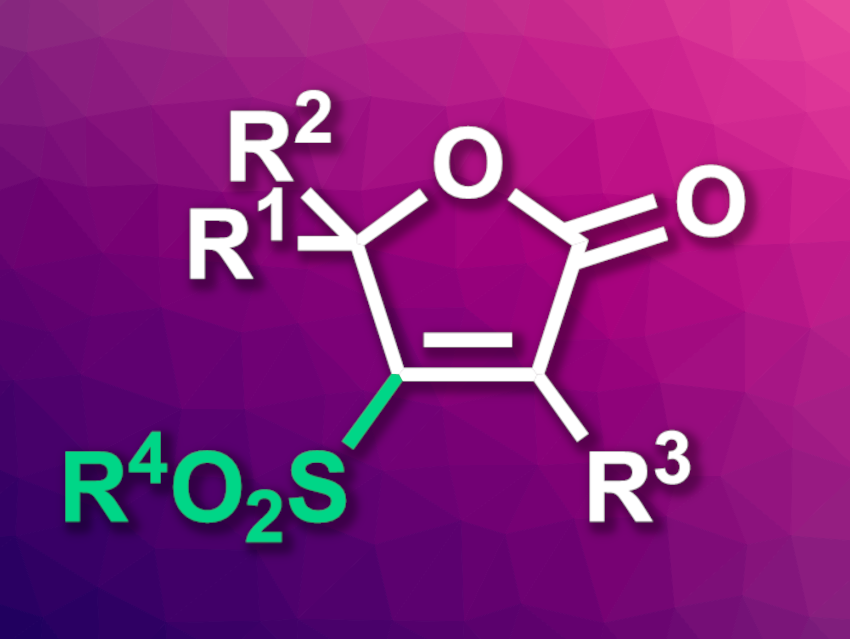
4-Sulfonylated furan-2(5H)-ones (general structure pictured) are found, e.g., in some natural products or compounds with biological activities. They can be synthesized, for example, via the oxidation of 2(5H)-furanonyl thioethers. Allenoic acids (R2C=C=C(R)CO2H) can serve as alternative starting materials for the synthesis of furan-2(5H)-one derivatives, but the direct sulfonylation of 2,3-allenoic acids with sulfonyl chlorides has remained challenging.
Jian Zheng, Shengming Ma, Zhejiang University, Hangzhou, China, and colleagues have developed a photocatalytic cyclization of 2,3-allenoic acids with sulfonyl chloride to give 4-sulfonylated furan-2(5H)-ones. The team reacted a variety of substituted 2,3-allenoic acids with a range of sulfonyl chlorides (RSO2Cl) in the presence of an iridium-based photocatalyst and TsOH·H2O as an additive under blue LED light (427 nm), using dichloromethane (DCM) as the solvent. The reactions were performed under a nitrogen atmosphere at room temperature.
Under these conditions, the desired 4-sulfonylated furan-2(5H)-ones were obtained in useful yields in most cases. Aryl sulfonyl chlorides gave better yields of the corresponding products than alkyl sulfonyl chlorides. The researchers also performed the reaction on a gram scale, obtaining the product in a yield of 57 %. They propose a reaction mechanism that involves a sulfonyl radical, which is generated via a light-induced single-electron transfer (SET) process and can react with the allenoic acid.
- Photocatalytic Chemoselective Cyclic Oxysulfonylation of 2,3-Allenoic Acids,
Yaqi Shi, Chunling Fu, Jian Zheng, Shengming Ma,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c01730