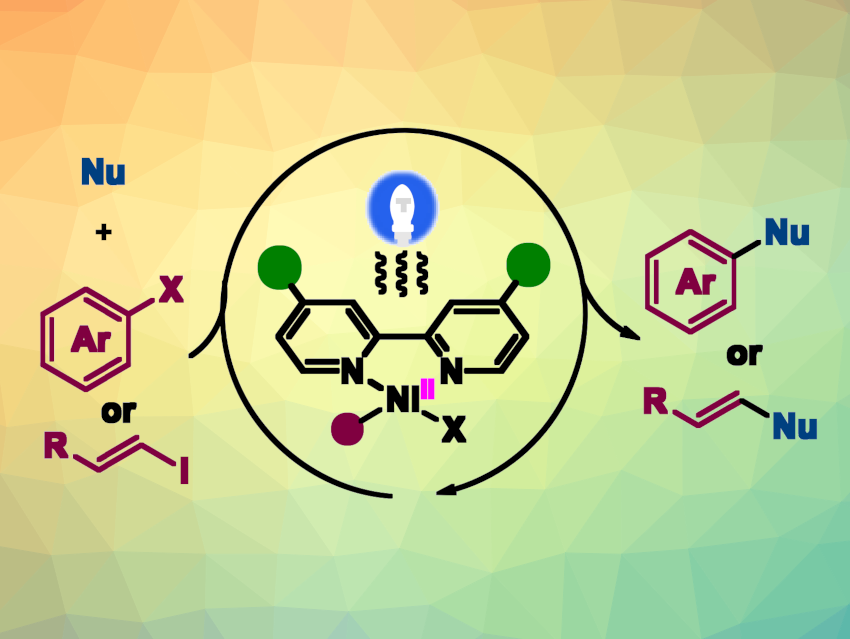
Carbon–heteroatom cross-coupling reactions are useful for the synthesis of, e.g., pharmaceutically active compounds or natural products. The commonly used palladium, copper, or nickel catalysts can require specially designed ligands, a strong base, or high temperatures to obtain satisfactory yields. Dual metal/photoredox catalysis can provide new pathways to access carbon–heteroatom bonds under mild conditions. However, the need for expensive noble metal-based photocatalysts limits its application in organic syntheses. Single metal catalysis, which performs the dual roles of light harvesting and organometallic catalysis, could provide a solution to this issue.
Luqing Lin, Dalian University of Technology, China, Hokkaido University, Sapporo, Japan, Shigeki Matsunaga, Hokkaido University, and colleagues have developed a visible-light-induced nickel-catalyzed carbon–heteroatom coupling (pictured) using commercially available 4,4′-di-tert-butyl-2,2′-bipyridine (dtbbpy) as the ligand. Diverse nitrogen, oxygen, and sulfur nucleophiles were successfully coupled with aryl/alkenyl iodides or bromides in tetrahydrofuran (THF) under blue LED light at 30 °C in the absence of an additional photocatalyst.
The desired C–N, C–O, and C–S coupling products were obtained in good to excellent yields. The reaction provides generality, robustness, practicality, and a broad substrate scope. It can be used for the late-stage functionalization of complex pharmaceuticals or natural products, as well as for gram-scale reactions. The researchers propose a NiI/NiIII catalytic cycle.
- Photo‐Induced Nickel‐Catalyzed Carbon‐Heteroatom Coupling,
Hang Luo, Guohua Wang, Yunhui Feng, Wanyao Zheng, Lingya Kong, Yunpeng Ma, Shigeki Matsunaga, Luqing Lin,
Chem. Eur. J. 2022.
https://doi.org/10.1002/chem.202202385