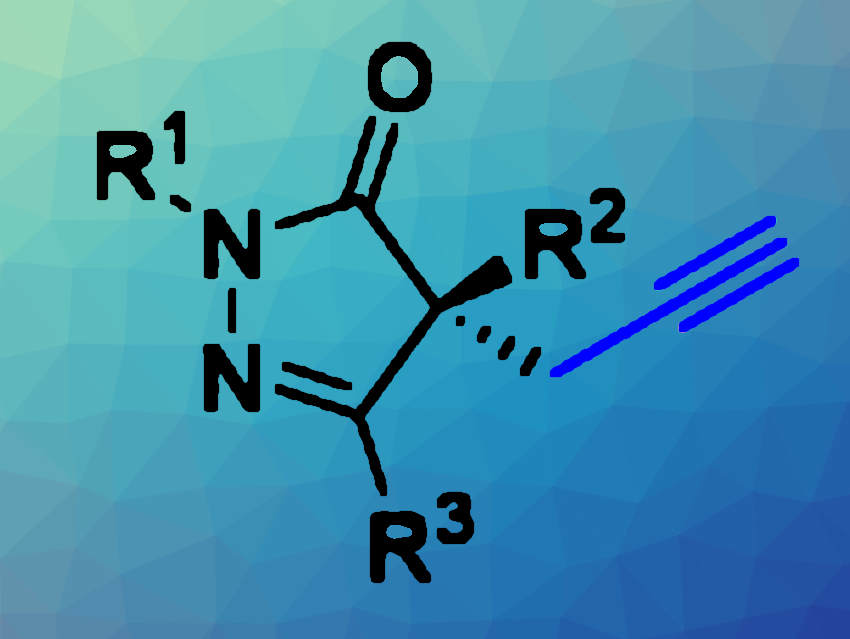
Enantioselective functionalizations are important, but sometimes challenging reactions in organic synthesis—for example, for drug discovery. For example, pyrazolones, five-membered heterocycles with two nitrogen atoms and a ketone functionality, are used in pharmaceutical chemistry. Thus, methods for, e.g., their enantioselective alkylation can be useful tools.
Yakun Wang, Tao Zhang, Xinxiang Medical University, Henan, China, and colleagues have developed an approach to the enantioselective alkylation of 4-substituted pyrazolones using phase-transfer catalysis (example product pictured). The team reacted different pyrazolones with electropholes such as propargyl chloride, propargyl bromide, 1-bromo-2-butyne, allyl bromide, or benzyl bromide in the presence of KF as a base and using PhCF3 as a solvent at room temperature. As a phase transfer catalyst, they employed an amide-based Cinchona alkaloid derivative with a triphenyl group.
Under these conditions, the desired alkylated products were obtained in mostly high to excellent yields and with good stereoselectivities. The team proposes that the catalyst provides this enenatioselectivity due to multiple hydrogen bonding interactions as well as the bulky triphenyl substituent, which interact with the substrate and ensure an attack at the desired side, respectively.
- Catalytic Enantioselective Propargylation of Pyrazolones by Amide-Based Phase-Transfer Catalysts,
Yakun Wang, Yingying Wang, Xiaoyu Du, Kaiting Zheng, Shuman Zhai, Suping Bai, Lizhen Fang, Tao Zhang,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c02441