To date, collected plastic waste is often used as a fuel substitute (“energetically recovered”) or mechanically recycled, usually by shredding the waste and processing the granules. In this process, recycled material is often more expensive than virgin plastics due to the complex collection, cleaning, and separation processes involved. Another option is cracking, in which the oil components from which the plastics were originally made are recovered. Polymers can also be chemically degraded, but this often results in chemicals that are cheaper to produce in their original form. A relatively new approach is upcycling, i.e., the conversion of plastic waste into chemicals with a higher market value, but, so far, often with limited use and, therefore, limited demand.
Alexis T. Bell, John F. Hartwig, and colleagues, University of California, Berkeley, and Lawrence Berkeley National Laboratory, CA, USA, have developed a method to effectively and selectively recycle the two most commonly used plastics – polyethylene (PE) and polypropylene (PP). A pair of catalysts breaks down the polymers and combines them with ethene to produce propene and isobutene, which can be used to make new plastics.
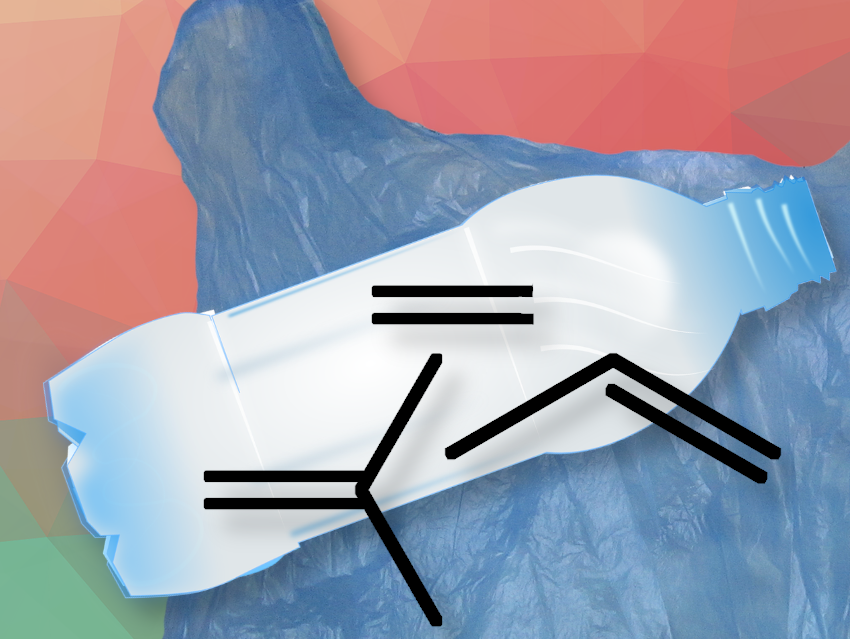
The researchers describe that the simple combination of tungsten oxide on silica and sodium on gamma alumina converts PE, PP, or a mixture of the two, including post-consumer forms of these materials, in the presence of ethene, into propylene or a mixture of propylene and isobutylene with over 90% yield at 320°C without the need for dehydrogenation of the starting polyolefins. First, the polymer is split into shorter chains with C=C double bonds by catalytic cracking. These double bonds are then cleaved by a metathesis reaction with ethene, resulting in smaller fragments with terminal C=C groups. An isomerization reaction then shifts the double bond by one position along the chain. After that, another ethene metathesis reaction breaks the C=C bond, forming a small molecule. This is propene from PE or a mixture of propene and isobutene from PP. This catalytic isomerization-metathesis cycle repeats continuously, shortening the polymer chains. The resulting mixture of ethene, propene, and isobutene could be separated using standard industrial cryogenic separation processes.
In a laboratory experiment, 90 mL of recyclate have been produced so far. It remains to be seen whether the process can be economically implemented on an industrial scale. If successful, it could significantly help address the growing plastic waste problem.
- Polyolefin waste to light olefins with ethylene and base-metal heterogeneous catalysts,
Richard J. Conk, Jules F. Stahler, Jake X. Shi, Ji Yang, Natalie G. Lefton, John N. Brunn, Alexis T. Bell, John F. Hartwig,
Science 2024, 385(6715), 1322-1327.
https://doi.org/10.1126/science.adq7316
Also of Interest

Chemolysis and pyrolysis are promising processes for cases in which mechanical recycling is not effective—an introduction and examples from Covestro