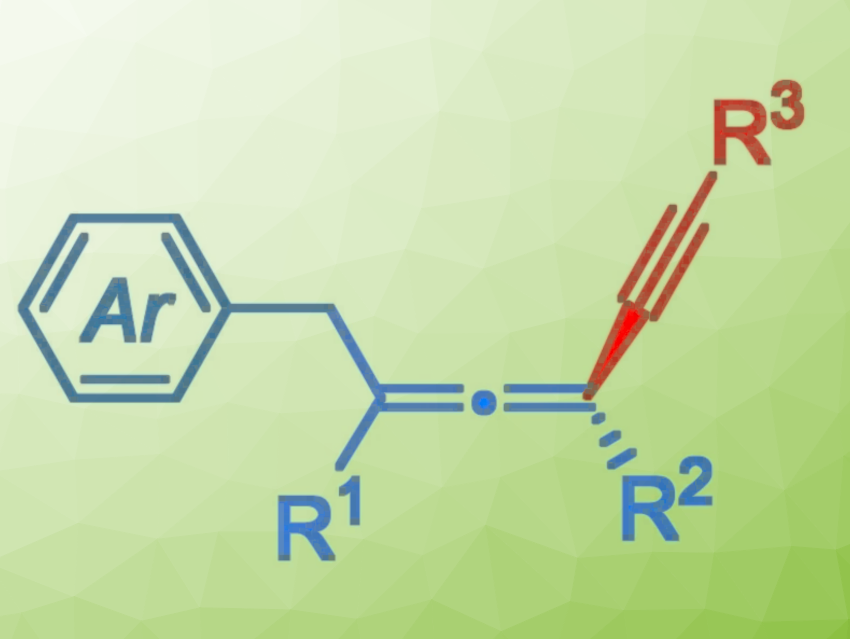
Axially chiral allenes are important structural elements found in natural products, biologically active molecules, and functional materials. The asymmetric 1,4-difunctionalization of 1,3-enynes is a concise and highly efficient strategy for the preparation of chiral allenes. However, this usually involves the reaction of an allenyl anion intermediate with electrophiles, which can limit the diversity of accessible allene products.
Guozhu Zhang, Rui Guo, Central China Normal University, Wuhan, China, and colleagues have developed a copper-catalyzed three-component asymmetric 1,4-aryl/alkynylation of 1,3-enynes to access chiral tetrasubstituted allenes (pictured). The team used alkynes as nucleophiles and diaryliodonium salts as aryl radical precursors. CuCN served as a catalyst, iminophenyl oxazolinylphenylamines (IPOPA, pictured below) as chiral ligands, Cs2CO3 as a base, and a mixture of diethyl ether and acetonitrile as the solvent. The reactions were performed at 0 °C over 48 h.
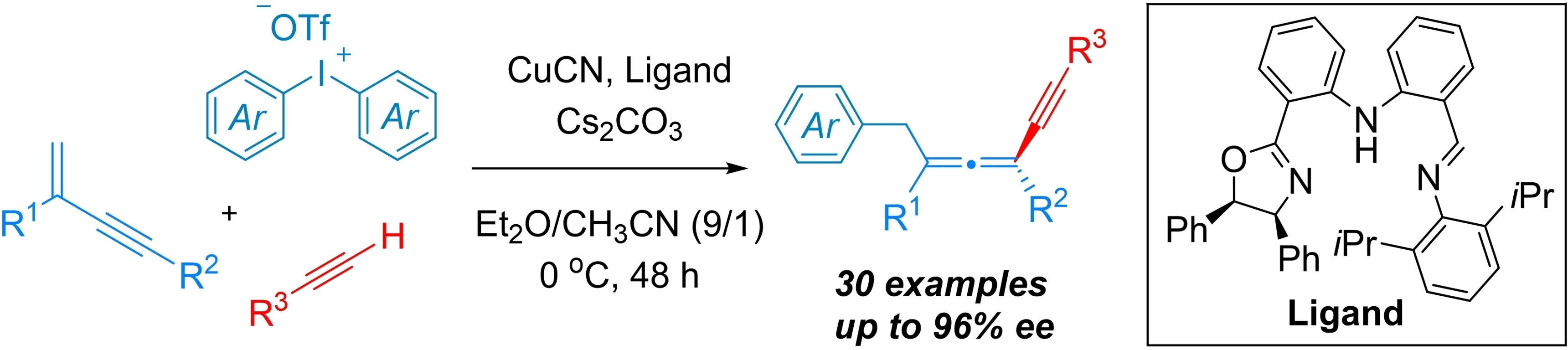
The reaction exhibits good compatibility with various alkynes, 1,3-enynes, and diaryliodonium salts, providing an effective method for constructing tetrasubstituted chiral allene compounds. The researchers propose a reaction mechanism that involves the addition of an aryl radical to the 1,3-enyne to generate an allenyl radical, followed by an enantioselective coupling with a copper(II) acetylide complex to give the allene products.
- Copper‐Catalyzed Asymmetric 1,4‐Aryl/Alkynylation of 1,3‐Enynes to Access Axially Chiral Tetrasubstituted Allenes,
Han Huang, Haiyan Zhang, Qiaoling Wang, Youwen Sun, Lei Su, Wenru Xu, Yi Ma, Sheng Kong, Guozhu Zhang, Rui Guo,
ChemCatChem 2023.
https://doi.org/10.1002/cctc.202300697