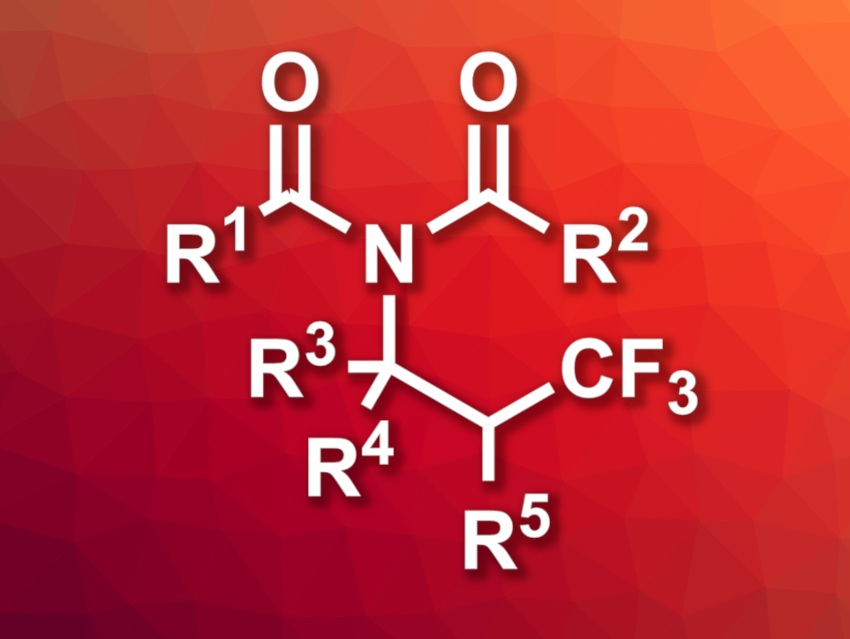
Imides, i.e., functional groups composed of two acyl groups bound to nitrogen, are found, for example, in polymers and pharmaceutically active compounds. Imides can be prepared using condensation reactions of amides with carboxylic acid derivatives. Multicomponent reactions can also be useful in this context. Trifluoromethyl groups can be useful, e.g., in pharmaceutical chemistry, but traditional trifluoromethylation reagents can be costly to synthesize. CF3Br, in contrast, is a low-cost fire suppressant that could serve as a cost-effective trifluoroalkylation reagent.
Yulai Hu, Northwest Normal University, Lanzhou, and Lanzhou University, China, and colleagues have developed a method for the synthesis of β-trifluoromethyl imides (general structure pictured) via photoinduced multi-component reactions, using CF3Br as a trifluoromethyl source. The synthesis is based on a four-component Ritter-type reaction of CF3Br, alkenes, carboxylic acids, and nitriles. The team used fac-IrIII(ppy)3 (ppy = 2-phenylpyridine) as a photocatalyst and K2CO3 as a base, and the reactions were performed under blue LED light.
Under these conditions, the researchers converted a wide range of alkenes, a variety of carboxylic acids, and different nitriles into the desired β-trifluoromethyl-substituted imides in moderate to high yields. The team demonstrated the utility of the approach by using it for the late-stage functionalization of pharmaceutically active compounds, as well as a reaction on the gram scale.
- Visible-Light-Induced Four-Component Ritter-Type Reaction: Access to β-Trifluoromethyl Imides,
Ransong Ma, Yuanyuan Ren, Zhoubin Deng, Ke-Hu Wang, Junjiao Wang, Danfeng Huang, Xiaobo Lv, Yulai Hu,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c01291