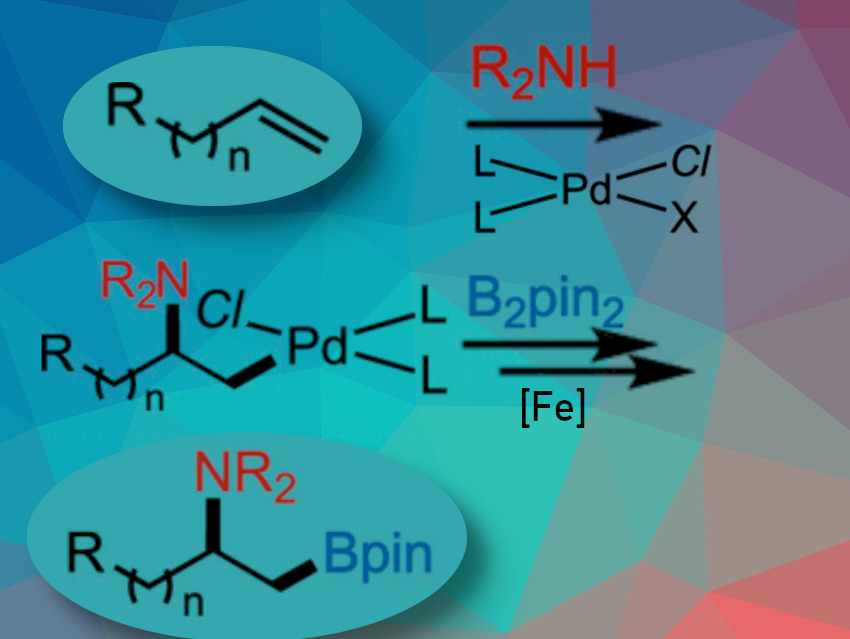
Aminoboration of simple alkenes with nitrogen nucleophiles is challenging. Catalytic conversion with palladium (aminopalladation followed by transmetalation with a diboron reagent) is difficult. The alkylpalladium(II) intermediate is unstable and rapidly tends to fall apart upon β-hydride elimination.
Kami L. Hull, University of Texas at Austin, USA, and colleagues have developed a palladium/iron co-catalyzed aminoboration to realize the directing-group-free aminoboration of terminal alkenes and norbornenes with common nitrogen nucleophiles and Bis(pinacolato)diboron (B2pin2). In their reaction, the transient alkylpalladium(II) undergos intermolecular transmetalation faster than intramolecular β-hydride elimination.
The iron cocatalyst is critical for achieving the desired reactivity. As a halophilic Lewis acid, it serves to release the transmetallation-active cationic alkylpalladium intermediate. In addition, it serves as a redox shuttle in the regeneration of the Pd(II) catalyst by reactivation of the nanoparticulate palladium.
- Palladium and Iron Cocatalyzed Aerobic Alkene Aminoboration,
Brittany L. Gay, Ya-Nong Wang, Shreeja Bhatt, Anika Tarasewicz, Daniel J. Cooke, E. Grace Milem, Bufan Zhang, J. Brannon Gary, Michael L. Neidig, Kami L. Hull,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c05790