To combat climate change, carbon dioxide emissions must be significantly reduced. A new electrochemical process offers a solution by directly splitting CO₂ into carbon and oxygen. According to a Chinese research team, this method could also generate oxygen underwater or in space without requiring strict pressure or temperature conditions.
Photosynthesis
Leafy plants are masters of the art of carbon neutrality: during photosynthesis, they convert CO₂ into oxygen and glucose. Hydrogen atoms play an important role as “mediators”. However, the process is not particularly efficient.
In addition, the oxygen produced does not come from the CO₂ but from the absorbed water. True splitting of CO₂ is not taking place in plants and also could not be achieved at moderate temperatures by technical means so far.
Directly Splitting CO₂ into C and O₂
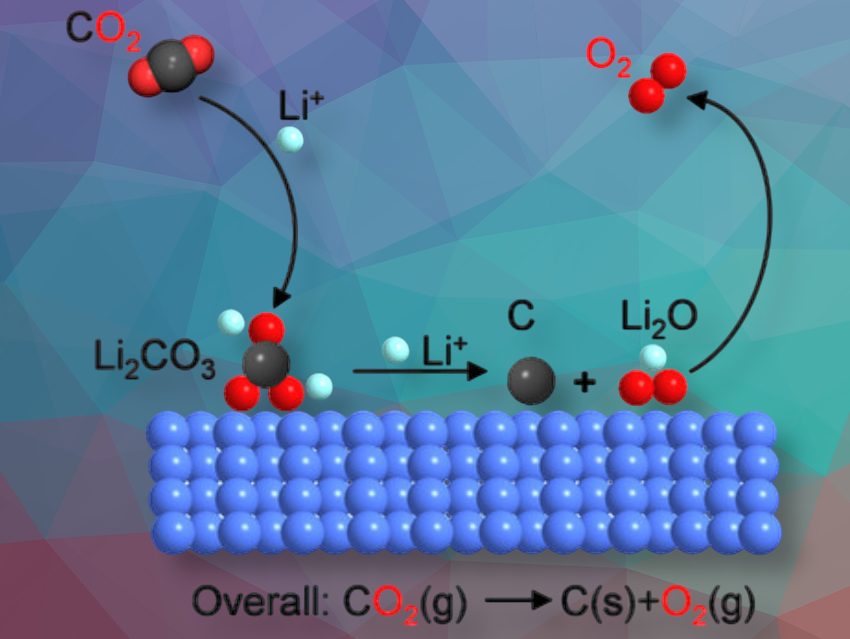
Ping He, Haoshen Zhou, and their team at Nanjing University, China, in collaboration with researcher from Fudan University, Shanghai, China, have achieved their goal to directly split CO₂ into elemental carbon and oxygen. Instead of hydrogen, the “mediator” in their method is lithium.
The team developed an electrochemical device consisting of a gas cathode with a nanoscale cocatalyst made of ruthenium and cobalt (RuCo) as well as a metallic lithium anode. CO₂ is fed into the cathode and undergoes a two-step electrochemical reduction with lithium. Initially, lithium carbonate Li₂CO₃ is formed, which reacts further to produce lithium oxide (Li₂O) and elemental carbon. In an electrocatalytic oxidation process, the Li₂O is then converted to lithium ions and oxygen gas (O₂).
O₂ from CO₂—A Step Toward Sustainable Breathing in Space and Underwater
An optimized RuCo catalyst enables an exceptionally high O₂ yield of over 98.6%, significantly surpassing the efficiency of natural photosynthesis. In addition to pure CO₂, successful tests were conducted with mixed gases containing varying CO₂ fractions, including simulated flue gas, a CO₂/O₂ mixture, and simulated Mars gas. Mars’ atmosphere is primarily composed of CO₂, though its pressure is less than 1% of Earth’s atmospheric pressure. The simulated Mars atmosphere, therefore, contained a mixture of argon and 1% CO₂.
If powered by renewable energy, this method could contribute to carbon neutrality. It also offers a practical, controllable way to produce O₂ from CO₂ with wide-ranging applications—from Mars exploration and oxygen supply in spacesuits to underwater life support, breathing masks, indoor air purification, and industrial waste treatment.
- Artificial Carbon Neutrality Through Aprotic CO₂ Splitting,
Wei Li, Xiaowei Mu, Sixie Yang, Di Wang, Yonggang Wang, Haoshen Zhou, Ping He,
Angewandte Chemie International Edition 2025.
https://doi.org/10.1002/anie.202422888