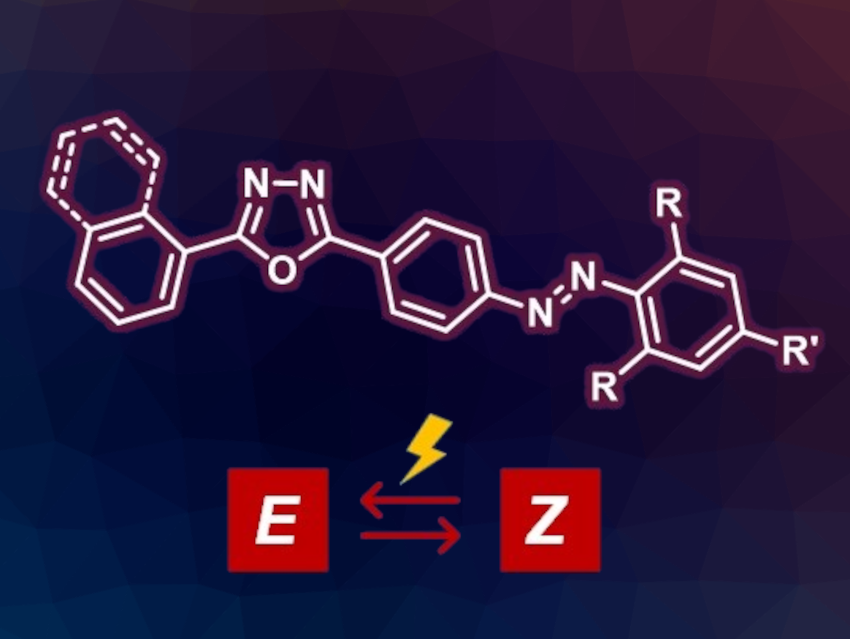
Azobenzenes can be used, e.g., as molecular photoswitches. These are compounds that can interconvert between two isomers in a controlled manner upon light irradiation. Suitable substituents can enhance the lifetime of the metastable isomer, and thus, improve the photoswitching behavior of azobenzenes. In addition, combining azobenzene derivatives with fluorophore units could be interesting, e.g., to build light-emissive switching molecules.
Mihaela Matache, University of Bucharest, Romania, and colleagues have combined fluorophores with azobenzene-based photoswitches. The team prepared novel oxadiazole-decorated azobenzenes (simplified structure pictured) via a synthetic strategy that involves amino-functionalized oxadiazoles as intermediates. The intermediates were used either to obtain electrophilic diazonium salts that can react with phenol derivatives or as nucleophilic partners in Baeyer–Mills reactions with nitroso-substituted derivatives. The team investigated the photoproperties of both the intermediates and the oxadiazole-decorated azobenzenes.
The amino-functionalized oxadiazole precursors were found to emit blue or green light. The oxadiazole-decorated azobenzenes lost their ability to emit light. However, light irradiation experiments in solution showed their robustness as molecular photoswitches: They were stable over repeated cycles of light irradiation and showed half-lives for the least stable isomers of hours to days.
- A Synthetic Approach for Oxadiazole‐Decorated Azobenzene Photoswitches,
Adela F. Dobre, Anamaria Hanganu, Ioana Nicolau, Codruta C. Popescu, Anca Paun, Augustin M. Mădălan, Cristina Tablet, Anca G. Mirea, Mihaela Matache,
ChemPlusChem 2023.
https://doi.org/10.1002/cplu.202300504