Thiiranes are the sulfur analogues of epoxides. Molecules with thiirane units can show interesting medicinal properties. Thiiranes have also been used as pesticides, herbicides, and in polymerization reactions. Although thiiranes form an important class of compounds, there is a limited number of approaches to their synthesis.
Christoforos G. Kokotos, Ierasia Triandafillidi, National and Kapodistrian University of Athens, Greece, and colleagues have developed a new method for the synthesis of thiiranes from alkenes. The approach uses an epoxidation reaction, followed by a ring-opening using thiourea (pictured below). The method features a cheap, easy-to-execute, organocatalytic protocol.
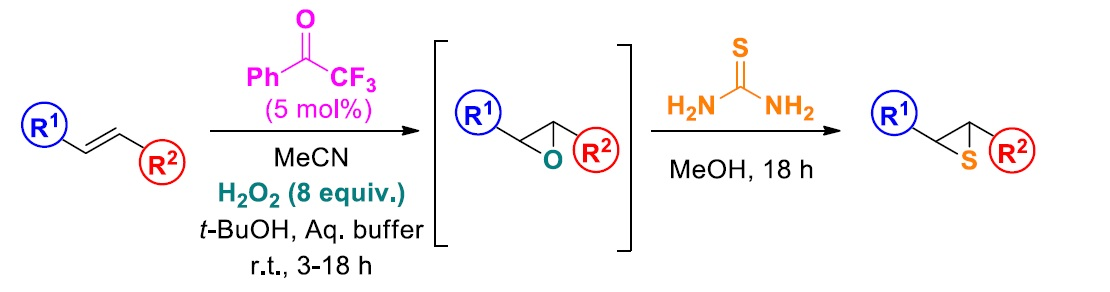
In the first step, the team used 2,2,2-trifluoroacetophenone as an organocatalyst and H2O2 as an oxidant to convert a variety of substituted aromatic styrenes and a range of other alkenes to the corresponding epoxide. In the second step, they used thiourea as a sulfur source under catalyst-free conditions to obtain the desired thiiranes.
The reaction works well with both aromatic and aliphatic substates. The desired products were obtained in moderate to excellent yields.
- Organocatalytic Synthesis of Thiiranes from Alkenes,
Anna Tsoukaki, Elpida Skolia, Ierasia Triandafillidi, Christoforos G. Kokotos,
Eur. J. Org. Chem. 2022.
https://doi.org/10.1002/ejoc.202200463