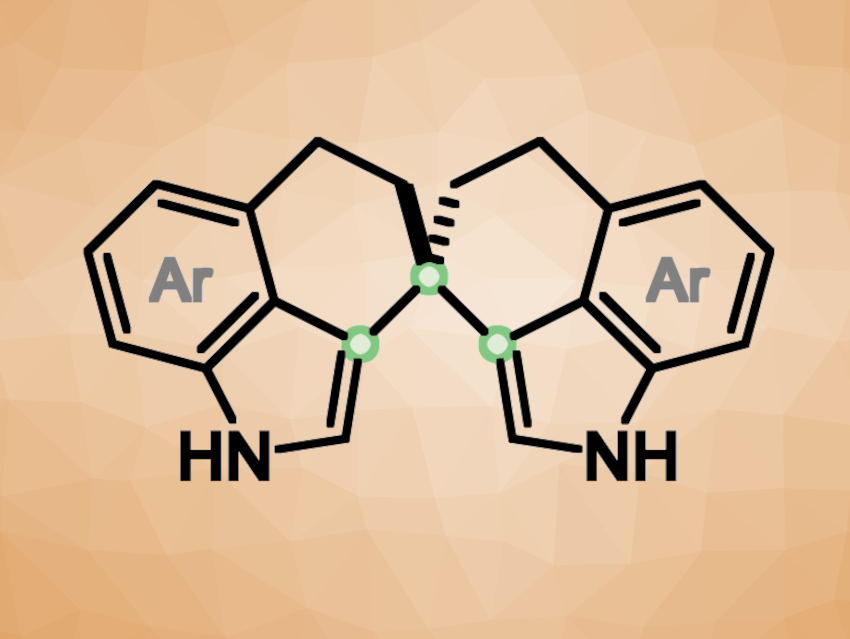
Axially chiral molecules are useful, e.g., in asymmetric catalysis. Atropisomeric biaryls, for example, have been well-explored and are often used as chiral catalysts and ligands. Axial chirality in molecules with a spirocyclic backbone can also be interesting in this context and play a complementary role to the “traditional” biaryl skeletons. However, the limited structural diversity of available spiro frameworks significantly restricts their range of applications.
Shao-Hua Xiang, Wei Lu, Bin Tan, Southern University of Science and Technology, Shenzhen, China, and colleagues have synthesized new axially chiral spiro-bisindole frameworks (general structure pictured). The team used an intramolecular dehydrative cyclization, catalyzed by a chiral phosphoric acid. They started from 1,5-di(1H-indol-4-yl)pentan-3-one derivatives, which were cyclized in the presence of a 1,1′-bi-2-naphthol (BINOL)-derived chiral phosphoric acid catalyst and MgSO4 as an additive. The reactions were performed in CHCl3 at 90 °C.
Under these conditions, the desired chiral, spirocyclic products were obtained in mostly good yields and with high enantioselectivities. The products can be further functionalized to provide access to a broader scope of products. The team also used the approach to obtain axially chiral fluorescent molecules with useful asymmetry factors and quantum fluorescence efficiencies.
- Organocatalytic Asymmetric Construction and Application of Axially Chiral Spiro‐bisindoles,
Bin Tan, Hao-Wen Zhao, Fei Jiang, Sihan Chen, Jingliang Hu, Shao-Hua Xiang, Wei-Yi Ding, Wei Lu,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202422951