Marina A. Petrukhina, University at Albany, NY, USA, Renana Gershoni-Poranne, Technion – Israel Institute of Technology, Haifa, and colleagues have studied the sequential addition of electrons to tetrabenzo[a,c,e,g]cyclooctatetraene (tetraphenylene) and found distinct structural changes, which complement textbook knowledge.
What is the main idea of your study?
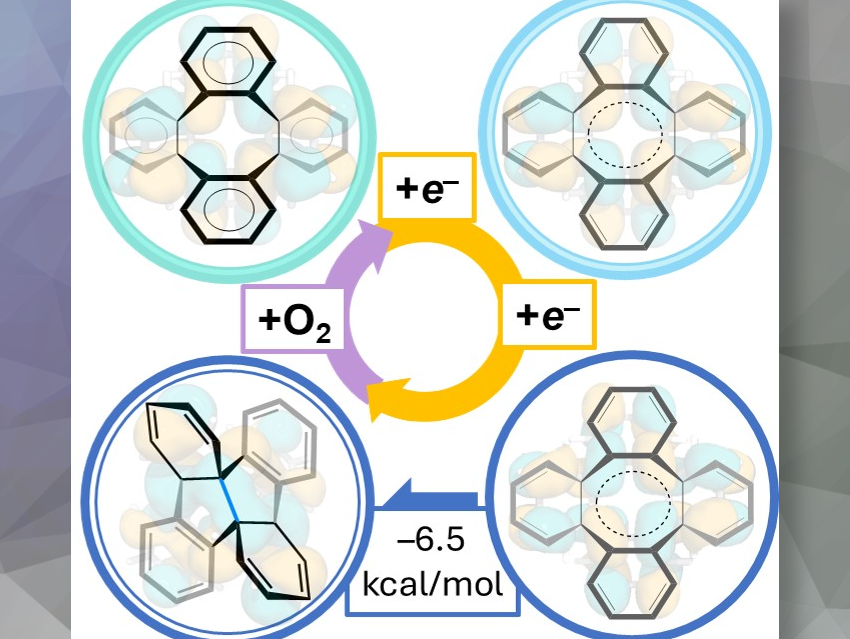
In this work, we investigated the chemical reduction of a cyclooctatetraene (COT) derivative, tetrabenzo[a,c,e,g]cyclooctatetraene (TBCOT), to reveal the influence of one- and two-electron addition to an eight-membered core. In principle, the dianionic COT is aromatic, with 10 π-electrons, and, therefore, prefers to adopt a planar geometry. However, the steric hindrance created by the presence of the four annulated benzene rings precludes planarization. We were intrigued to see how the molecule would deal with these opposing effects.
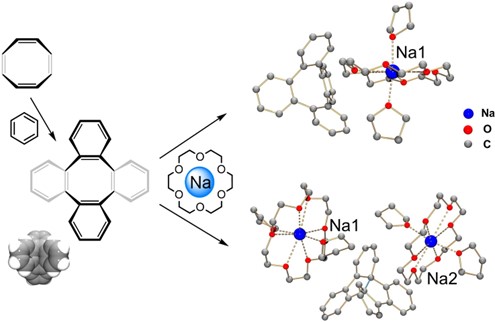
We successfully isolated single crystals of the sodium-reduced products of TBCOT and managed to characterize the two stepwise reduced products—meaning, both the anion and dianion of the TBCOT—using X-ray diffraction analysis and spectroscopic methods [1]. Solid evidence from single crystal X-ray diffraction analysis allowed us to study the geometric changes that the molecule underwent.
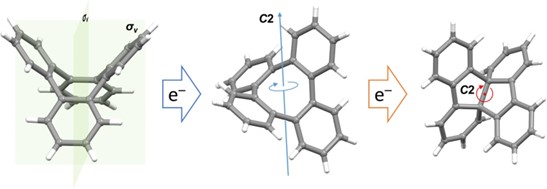
It showed that the addition of the first electron only slightly distorts the central eight-membered ring and reduces the molecular symmetry. However, the addition of the second electron leads to a unique transformation of the COT core, in which a new C–C single bond is formed, thus turning the eight-membered ring into a bicyclic structure. Lastly, DFT calculations confirmed that the core rearrangement occurs only after the second electron addition, and that the driving force for the core transformation is to maximize charge separation.
Why are you interested in this?
This work aimed to answer a fundamental and longstanding question in the organic chemistry and aromaticity communities. Organic textbooks often mention the well-known conversion of the tub-shaped, non-aromatic COT to the planar, aromatic COT2– ring with 10π-electrons, which has enabled a broad variety of organometallic chemistry of main group, d-, and f-elements. However, even after many decades of research, the structure of the mono-reduced COT•– remained unclear. What happens upon the addition of one electron? And what happens to a COT core fused within a π-system that prevents planarization?
To address these questions, we selected a COT derivative, namely TBCOT, which can be readily prepared and purified for chemical reduction reactions.
What is new and cool about it?
To the best of our knowledge, this is the first experimental validation of a monoanionic derivative of the archetypal COT system. In addition to the structural characterization detailed in the manuscript, we obtained new computational insights into the stepwise electron addition to TBCOT. Our computational analysis of the changes in aromaticity, charge distribution, and energetic stability that accompany the chemical reduction process allowed us to form a deeper understanding of this unique process.
Our X-ray crystallographic studies revealed a small asymmetric distortion of the saddle-shaped core upon one-electron uptake, while the doubly-reduced product showed a dramatic transformation into a twisted conformation with a new C–C bond, effectively separating the central eight-membered ring into two fused five-membered rings. This structural change is reversible, as demonstrated by NMR spectroscopy.
What is the main significance of your results?
This research provides the first experimental evidence addressing the long-standing question of whether one electron is sufficient to induce structural changes in the COT core.
In this work, we successfully characterized the first single-crystal structure of the mono-reduced COT derivative and evaluated the influence of one electron addition to the COT core – which resulted in a slightly disturbed molecular symmetry but left the overall tub-shaped structure. Only after the addition of the second electron, a dramatic core transformation is observed.
In addition to the new information we have gleaned about this prototypical system, these findings may shed light on various systems in which COT-based derivatives are used as ligands for catalysts. Such structural changes may influence the ligand’s ability to participate in different oxidative/reductive catalytic steps.
Is there a specific application you imagine?
As demonstrated by in situ spectroscopic investigation, this two-fold reduction process, along with the core transformation, is fully reversible, which points to the potential application as a charge-dependent molecular switch.
What part of your work was the most challenging?
The preparation and isolation of the radical anion TBCOT•– provided the greatest synthetic challenge.
Most of the time, when a hydrocarbon molecule is reduced to different reduction stages, the individual species exhibit distinct and characteristic colors. However, in this case, the solutions of the mono- and dianions have a very similar color tone, so after multiple unsuccessful attempts to capture the elusive intermediate, we had to switch to a different procedure – a comproportionation reaction.
By mixing the solution of neutral TBCOT and TBCOT2– in 1:1 ratio, the charge transfer process resulted in the formation of the radical anion TBCOT•– which eventually led to the successful product isolation as a single crystalline material.
Anything else you would like to add?
We would like to add that this result stems from successful international collaborations between experimental and computational groups, and that four authors (including the two corresponding authors) are female researchers from different countries.
Thank you very much for these insights into your work.
The paper they talked about:
- What a Difference an Electron Makes: Structural Response of Saddle-Shaped Tetraphenylene to One and Two Electron Uptake,
Yikun Zhu, Zheng Zhou, Zheng Wei, Alexandra Tsybizova, Renana Gershoni-Poranne, Marina A. Petrukhina,
ChemistryEurope 2024.
https://doi.org/10.1002/ceur.202400055

Marina A. Petrukhina is Carla Rizzo Delray Professor of Chemistry at the Department of Chemistry & ETEC at the University at Albany, NY, USA.

Renana Gershoni-Poranne is Professor at the Schulich Faculty of Chemistry, Resnick Sustainability Center for Catalysis, Technion – Israel Institute of Technology in Haifa, Israel.