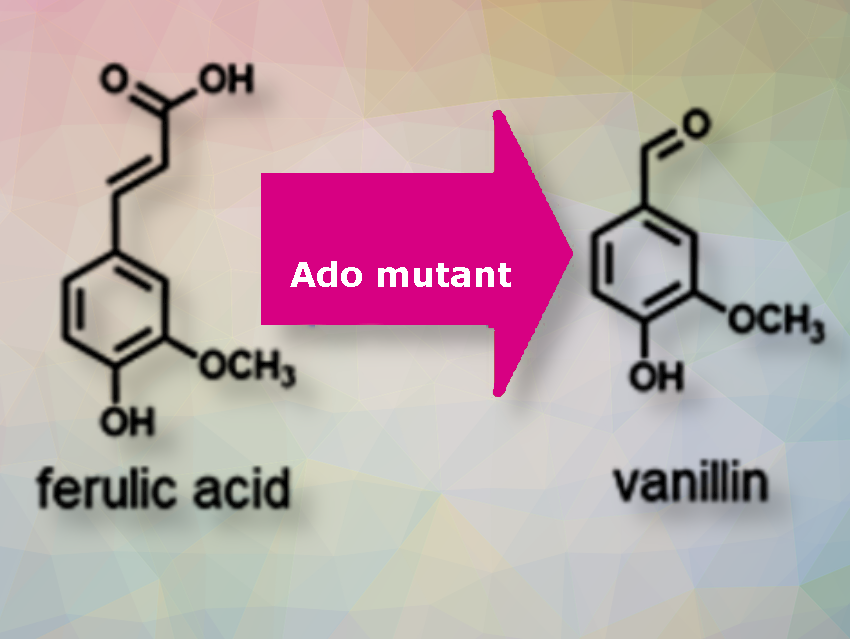
Vanillin is one of the world’s most important flavor and fragrance compounds used in foods and cosmetics. In plants, vanillin is biosynthesized from ferulic acid by the hydratase/lyase-type enzyme VpVAN. Laboratory biosynthesis of vanillin from plant-derived VpVAN yields very small amounts. The demand for natural vanilla extract remains high, also due to its superior flavor compared to chemically derived vanilla essences. The limited supply of vanilla plants and the climatic limitations for growing vanilla plants have resulted in high prices for natural vanilla extract.
Toshiki Furuya and colleagues, Tokyo University of Science, Japan, have used genetic engineering to modify the enzyme Ado, an oxidase enzyme that adds an oxygen atom to the substrate isoeugenol, enabling it to interact with ferulic acid and efficiently produce vanillin. Ferulic acid is abundantly available from agricultural wastes such as rice and wheat bran.
Using structural modeling analysis, the researchers were able to predict amino acid changes in Ado that would allow it to interact with ferulic acid. This new process is simple, sustainable, and economically scalable because it requires only the mixing of ferulic acid and air at room temperature, resulting in vanillin production on a gram scale per liter of reaction solution, with higher catalytic efficiency and affinity than the wild-type enzyme.
The team discovered that the single enzyme can catalyze not only the production of vanillin from ferulic acid, but also the synthesis of other aldehydes from p-coumaric acid, sinapinic acid, and coniferyl alcohol. These findings suggest that the approach used in this study can greatly expand the range of substrates available to the dioxygenase family of enzymes.
- Engineering a coenzyme-independent dioxygenase for one-step production of vanillin from ferulic acid,
Shizuka Fujimaki, Satsuki Sakamoto, Shota Shimada, Kuniki Kino, Toshiki Furuya,
Applied and Industrial Microbiology 2024.
https://doi.org/10.1128/aem.00233-24