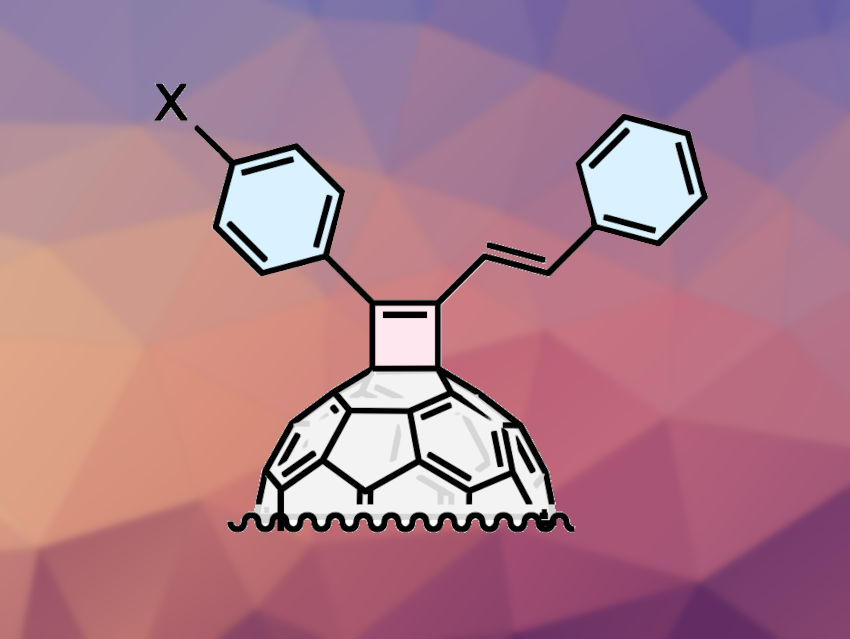
The physicochemical properties of fullerene-based materials vary depending on the type and mode of addition of functional groups. Thus, the development of fullerene derivatives with new structures can be useful in materials science.
Michio Yamada, Tokyo Gakugei University, Japan, and colleagues have developed an efficient one-step strategy for the synthesis of cyclobutene-annulated fullerene derivatives (cyclobutenofullerenes, simplified example pictured). The team reacted the fullerene C60 with selected secondary propargylic phosphates with aryl-terminated alkyne moieties in the presence of CuCl. The reactions were carried out in 1,2-dichlorobenzene (1,2-DCB) at a temperature of 180 °C.
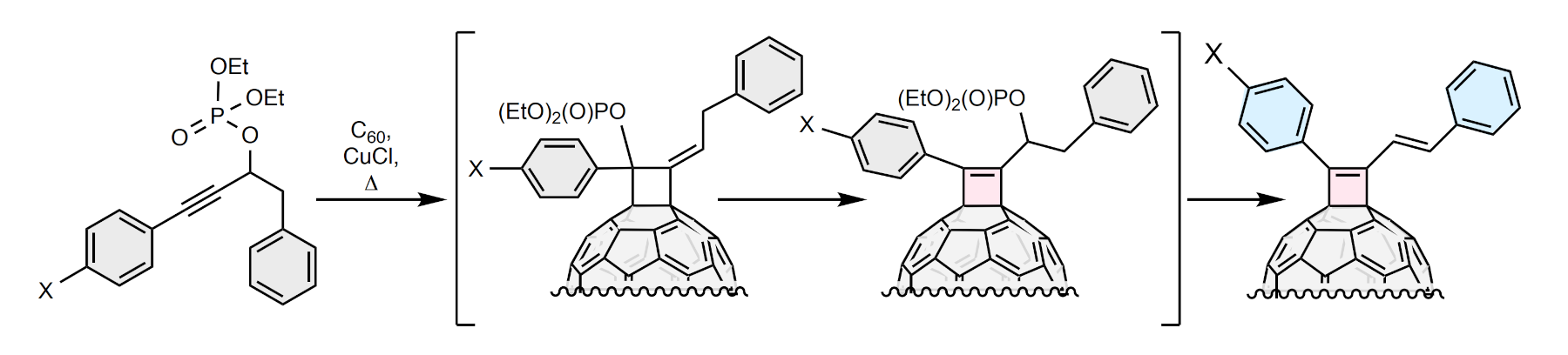
The researchers found that a variety of propargylic phosphates were able to react with C60, producing cyclobutenofullerenes with a yield of up to 76 % based on the amount of C60 consumed. The team proposes that the formation of cyclobutenofullerenes occurs through a cascade of reactions, including an 1,3-migration in the propargylic phosphate leading to a formal [2 + 2] cycloaddition of C60 and an allene, followed by an additional 1,3-migration and the 1,2-elimination of the phosphodiester moiety.
- One‐Step Synthesis of Cyclobutene‐Annulated Fullerenes Through a Cascade Reaction,
Michio Yamada, Yuta Uokawa, Shino Sasaki, Naohiro Iha, Yoshihisa Hashimoto, Yuya Nagasaki, Yutaka Maeda, Mitsuaki Suzuki,
Eur. J. Org. Chem. 2023.
https://doi.org/10.1002/ejoc.202300633