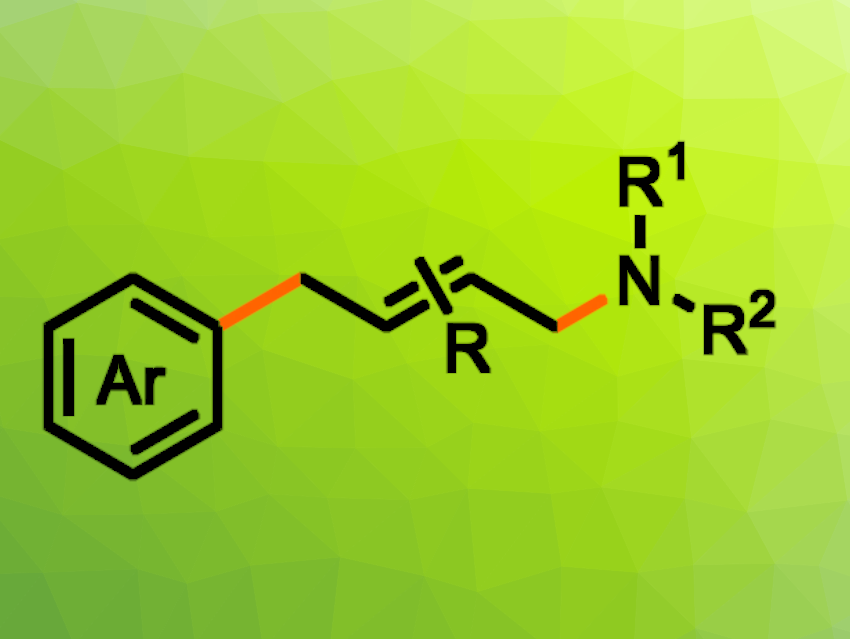
Palladium-catalyzed reactions that create new C–C or C–N bonds are widely used, both in research and in the chemical industry. These types of reactions can involve, e.g., aryl halides, alkenes, and amines. Nevertheless, a three-component reaction coupling aryl halides, butadienes, and amines to give aryl-substituted allylamines has remained elusive so far.
Tobias Ritter, Max Planck Institute for Coal Research, Mülheim an der Ruhr, Germany, and colleagues have developed a method for the visible-light-induced, three-component palladium-catalyzed 1,4-aminoarylation of butadienes with readily available aryl halides and aliphatic amines (pictured). The team used Pd(PPh3)4 as the catalyst together with rac-BINAP, K2CO3 as a base, and MeCN as the solvent. The reactions were performed under blue LED light.

Under these conditions, the desired allylamines were obtained in moderate to high yields and with high E-selectivity. The reaction is chemo-, regio-, and stereoselective and shows a wide substrate scope and good functional group compatibility. The team proposes a mechanism that involves the generation of an aryl radical in the presence of excited Pd(0). Overall, the developed process allows for the efficient synthesis of complex aryl-substituted allylamines from commercially available reagents in one step.
- 1,4‐Aminoarylation of Butadienes via Photoinduced Palladium Catalysis,
Yuan Cai, Gaurav Gaurav, Tobias Ritter,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202311250