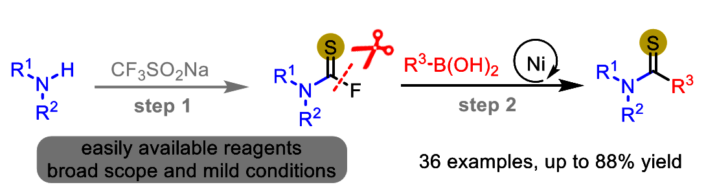
Wen-bin Yi and colleagues, Nanjing University of Science and Technology, China, have developed an efficient one-pot synthesis of thioamides. This approach involves generating thiocarbamoyl fluorides in situ from amines and then coupling them with boronic acids, catalyzed by nickel with PCy3 as a ligand. PCy3 is tricyclohexylphosphine (P(C₆H₁₁)₃) The process operates under mild conditions and allows for the synthesis of various thioamides. It also allows late-stage modifications.
As pictured schematically below, thiocarbamoyl fluorides are generated from the reaction of amines, CF3SO2Na, triphenylphosphine and chlorodiphenylphosphine. Then, oxidative addition of thiocarbamoyl fluorides and Ni0(PCy3)2 delivers a two-valent nickel species. Next, transmetalation of this nickel secies with arylboronic acids provides an intermediate, which gives the final products and regenerates Ni0(PCy3)2 through reductive
elimination.
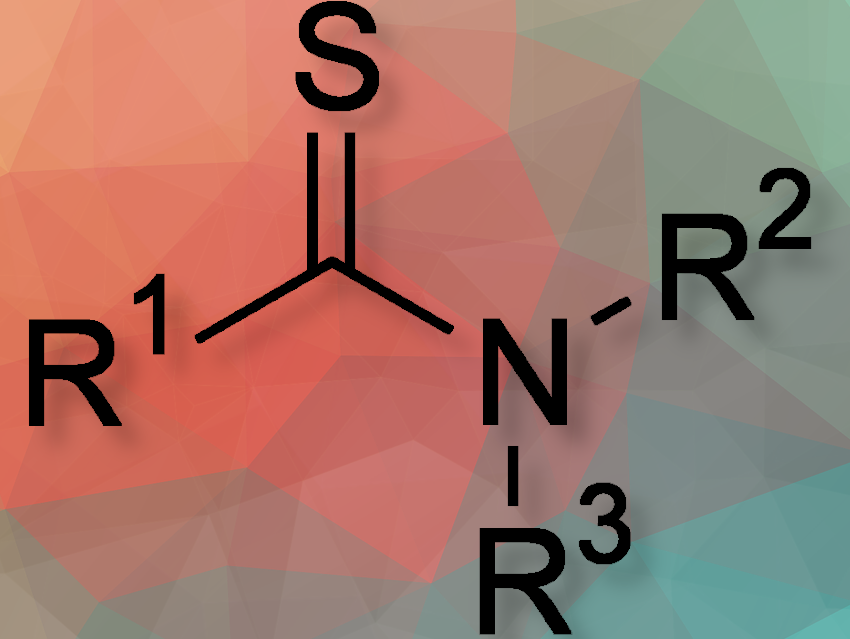
Thioamides, significant for their pharmaceutical and biological applications, have traditionally been difficult to synthesize due to low efficiency and selectivity in existing methods. This new method, the researchers say, addresses these issues, offering a broad substrate scope and good functional group tolerance.
- One-pot Synthesis of Thioamides via Nickel-Catalyzed Coupling of Thiocarbamoyl Fluorides and Boronic Acids,
Yasir Mumtaz, Haonan Xiang, Jahangir Khan, Shuaishuai Liang, Wen-bin Yi,
Eur. J. Inorg. Chem. 2024.
https://doi.org/10.1002/ejoc.202400742