Aziridines (three-membered heterocycles with one nitrogen atom) are important structural motifs that can be found in a range of biologically active compounds. They can also be useful intermediates in organic synthesis. New methods for the synthesis of aziridines are, thus, interesting research targets, Saccharin-derived cyclic ketimines (SDCIs, pictured below on the left) are useful reactive building blocks. A formal [2+1] cycloaddition reaction of SDCIs with sulfur ylides could give aziridines.
Chao Lin, Yantai Institute of Materia Medica, Bohai Rim Advanced Research Institute for Drug Discovery, Yantai, China, and colleagues have developed such a reaction. The team started from a range of SDCIs with different substituents and reacted them with sulfur ylide derivatives in MeCN (pictured below).
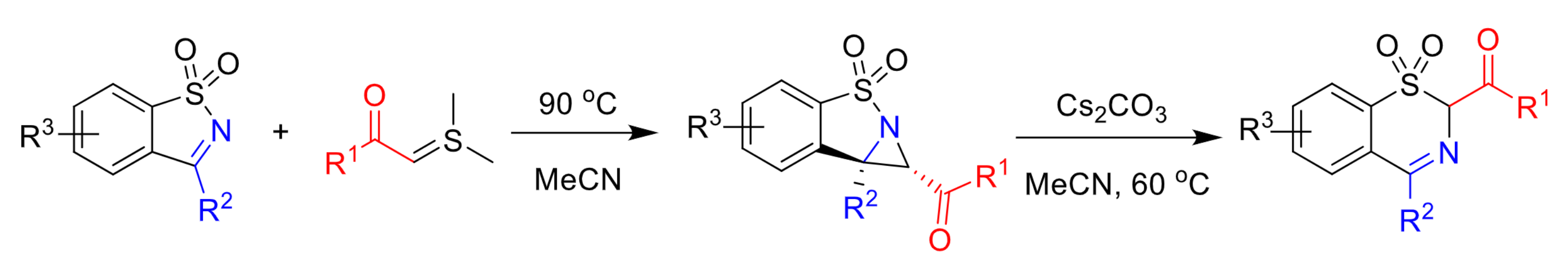
The desired multisubstituted fused aziridines were obtained in moderate to excellent yields. A reaction on the gram scale gave a yield of 92 %. The team found that the products can be readily converted to benzothiazines (pictured above on the right) in the presence of a base via a rearrangement reaction, demonstrating their synthetic utility. The method provides high diastereoselectivity, high atom economy, a broad substrate scope, and an operationally simple procedure.
- A One‐Pot Approach to Multisubstituted Fused Aziridines: Formal Intermolecular [2+1] Cycloaddition Reaction between Saccharin‐Derived Cyclic Ketimines and Sulfur Ylides,
Chao Lin, Mao-Chang Wang, Peng Wei, Qi Xing, Chen-He Liu,
Eur. J. Org. Chem. 2023.
https://doi.org/10.1002/ejoc.202300300
![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)


