The development of multifunctional materials that can serve more than one purpose, e.g., in energy-related applications, is an interesting research target. Applications such as electrocatalytic water splitting reaction and energy storage, for example, are important for the transition to renewable energy technologies. They require suitable electrocatalysts and electrode materials. Bismuth molybdenum oxide (BMO) is an interesting candidate for such electrochemical processes.
Sethumathavan Vadivel, SRM Institute of Science and Technology, Chennai, India, and colleagues have investigated the use of the BMO phase α-Bi2Mo3O12 for different applications in electrocatalysis and energy storage. The team synthesized the compound using a simple solvothermal method and evaluated the effects of calcination and base etching on its properties.
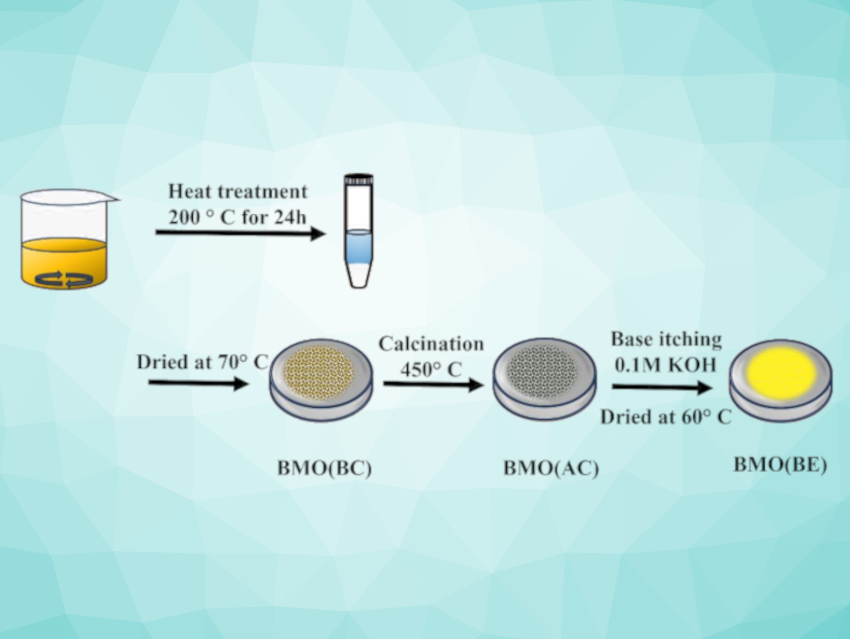
The researchers first reacted bismuth(III) nitrate pentahydrate with ammonium molybdate in the presence of hexamine in ethylene glycol at 200 °C. The product was separated using centrifugation and dried at 70 °C. The resulting product—BMO (BC) for “before calcination”—was calcined at 450 °C to give the modified form BMO (AC). In addition, a base-etched form, BMO (BE), was obtained by treating BMO (AC) with a KOH solution.
The team found that BMO (AC) showed the best performance for the hydrogen evolution reaction (HER) in electrocatalytic water splitting, while BMO (BC) was most effective for the oxygen evolution reaction (OER). The base-etched BMO (BE) is suitable for applications in supercapitors, but showed lower HER and OER activities. These different properties can be attributed to, e.g., different crystallinities and specific surface areas in the differently treated samples. Overall, the work shows the potential of α-Bi2Mo3O12 in different forms as a functional material for energy storage and conversion.
- α‐Bi2Mo3O12: A Dual‐Functional material for electrocatalytic water splitting and supercapacitor applications,
P. Sujita, S. Swetha, Sethumathavan Vadivel,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202402645