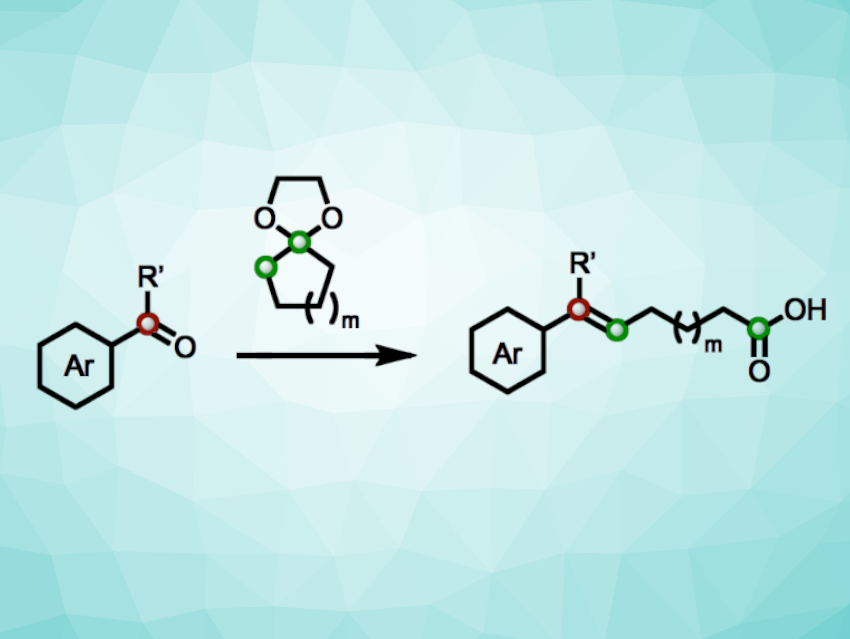
Methods for modifying the carbon backbone of organic compounds are important in synthesis. Such “skeletal editing” methods are useful for tailoring molecular properties, e.g., in pharmaceutical chemistry or materials science. Cyclic ketones, for example, can serve as readily available synthetic precursors for a variety of complex molecules. Often, skeletal editing on cyclic ketones involves ring expansions or ring contractions, while ring-opening reactions have been less well-explored.
Thanh Vinh Nguyen, University of New South Wales, Sydney, Australia, and colleagues have developed a method for the olefination of aromatic carbonyls using ketals of cyclic ketones to obtain valuable unsaturated carboxylic acids with extended carbon chains. The approach is based on a cascade reaction involving enolization to give a “hemiketal enol” intermediate as well as [2+2] cycloaddition and [2+2] cycloreversion steps. The team reacted a range of aromatic aldehydes with cycloalkanone ketals of different ring sizes (e.g., ketals of cyclobutanones, cyclopentanones, or cyclohexanones) in the presence of triflic acid and nitromethane at 40–60 °C, in some cases followed by hydrolysis with methanol and an aqueous NaOH solution.
The desired unsaturated carboxylic acids were obtained in moderate to high yields. The cyclic ketone precursors can be easily functionalized into substituted derivatives, and these substituents are transferred to the carboxylic acid products. For example, the method provides convenient access to fluorine-containing organic compounds that can be useful in drug development.
- Olefination of Aromatic Carbonyls via Site‐Specific Activation of Cycloalkanone Ketals,
Tuong Anh To, Thanh Vinh Nguyen,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202317003