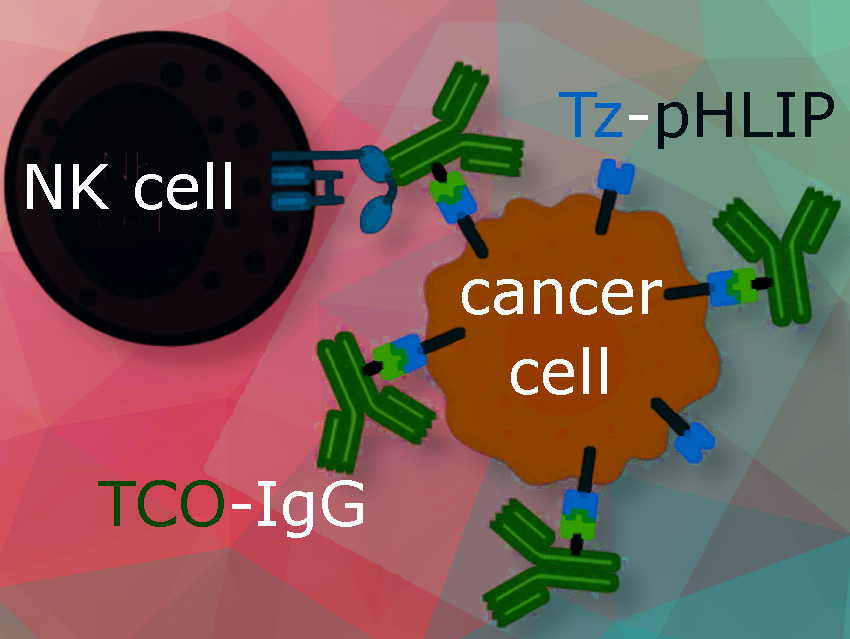
Emily Ankrom, Lehigh University, Bethlehem, PA, USA, and colleagues have developed a method using click chemistry to attach antibodies to the surface of cancer cells, thereby enhancing the ability of natural killer cells to target and destroy these cells.
What did you do?
We developed a new method using a unique peptide called the pH-low insertion peptide (pHLIP) to display antibodies on tumor cells and attract natural killer (NK) cells to destroy them. pHLIP peptides accumulate in tumors by integrating into cancer cell membranes under the acidic conditions in tumor environments.
To facilitate the attachment of antibodies to cancer cells via pHLIP, we equipped both pHLIP and antibodies with specific reactive sites, enabling their linkage through tetrazine-trans-cyclooctene ‘click’ chemistry.
Why are you doing this?
We aim to bypass the need for tumor-specific biomarkers commonly used by many anti-cancer immunotherapies. These strategies will only be effective if the cancerous cells produce high levels of specific biomarkers—molecules that allow the treatment to preferentially accumulate in tumors while sparing healthy tissues.
However, the highly variable nature of expression of these markers makes it challenging to selectively target only cancer cells, leading to potential side effects and difficulty in treating tumors without identifiable markers. To overcome this, we use pHLIP to enrich tumor cells with unique attachment points for antibodies, bypassing the need for tumor-specific markers.
How does this work in more detail?
We’ve leveraged click chemistry to create strong, lasting bonds between antibodies and tumor cell surfaces.
Typically, therapeutic antibodies bind to tumor cells through relatively weak and reversible interactions with antigens. However, we used the super-efficient tetrazine-trans-cyclooctene (Tz-TCO) ligation—currently one of the fastest bioorthogonal click chemistry reactions available—to help therapeutic antibodies to attach more robustly to tumor cells.
This method anchors antibodies to tumor cell membranes through a reaction that forms an irreversible covalent bond, potentially boosting the antibody’s ability to trigger a robust immune response from NK cells.
Our approach stands out not only for its effectiveness but also for its versatility and broad applicability across different types of solid tumors. Unlike other strategies that target specific tumor antigens, pHLIP homes in on the acidic environment found around most solid tumors. This unique trait makes our strategy versatile and widely applicable across different types of solid tumors.
What are your key findings?
Our study showed that we could specifically label cancer cells with IgG antibodies using tetrazine-TCO click chemistry, with pHLIP serving as a membrane anchor. This decoration of cancer cells with IgGs enabled activation of the body’s immune cells, leading to a strong cytotoxic (cell-killing) response in the presence of natural killer (NK) cells.
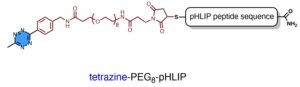
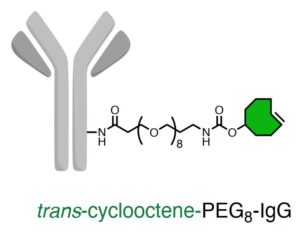
This approach causes NK cells to kill tumor cells through a process known as antibody-dependent cell-mediated cytotoxicity (ADCC), a powerful immunotherapy tactic used in monoclonal antibody treatments.
What are the next steps in your research?
Given pHLIP’s proven ability to target tumors in animals, we anticipate that our strategy will enhance tumor immunogenicity and reduce tumor volume. This could be effective on its own or as a supplementary treatment.
We aim to compare the effectiveness of traditional antigen-antibody binding versus permanent attachment in animal models. Additionally, we plan to test pHLIP variants that have shown higher tumor specificity than the original peptide used in this research. We also consider testing smaller antibody fragments instead of the complete antibodies to see if they improve targeting and penetration into the tumor’s environment.
What part of your work was the most challenging?
The greatest challenge of this work was carefully designing the necessary in vitro assays to test our hypothesis.
We had to mimic the acidic tumor microenvironment to selectively label cultured cells with pHLIP. These labeled cells then served as the substrate on which the subsequent click reaction takes place. So we had to precisely control and optimize several factors such as timing, concentrations, and temperature to ensure the cells weren’t too stressed before introducing the NK effector cells. As their name implies, NK cells are highly effective at destroying tumor cells, sometimes making it difficult to tell if the killing observed was due to our treatment or just the NK cells’ natural activity.
Despite these challenges, we demonstrated that our treatment specifically targets cells based on their pH levels, leading to cell death.
Thank you very much for these insights.
The article they talked about
- Selective Recruitment of Antibodies to Cancer Cells and Immune Cell-mediated Killing via In Situ Click Chemistry,
Emily T. Ankrom, Brianna Dalesandro, Marcos M. Pires, Damien Thévenin,
ChemMedChem 2024.
https://doi.org/10.1002/cmdc.202400356
Emily T. Ankrom is a graduate student in the group of Professor Damien Thévenin, Lehigh University, Bethlehem, PA, USA.