Electrocatalytic water splitting could be a part of the transition to renewable energy technologies, since hydrogen that is produced using sustainable electricity can serve as an environmentally friendly fuel. This approach requires suitable catalysts for the electrochemical hydrogen evolution reaction (HER). Without an efficient catalyst, the slow kinetics of the HER severely limit the rate of hydrogen production.
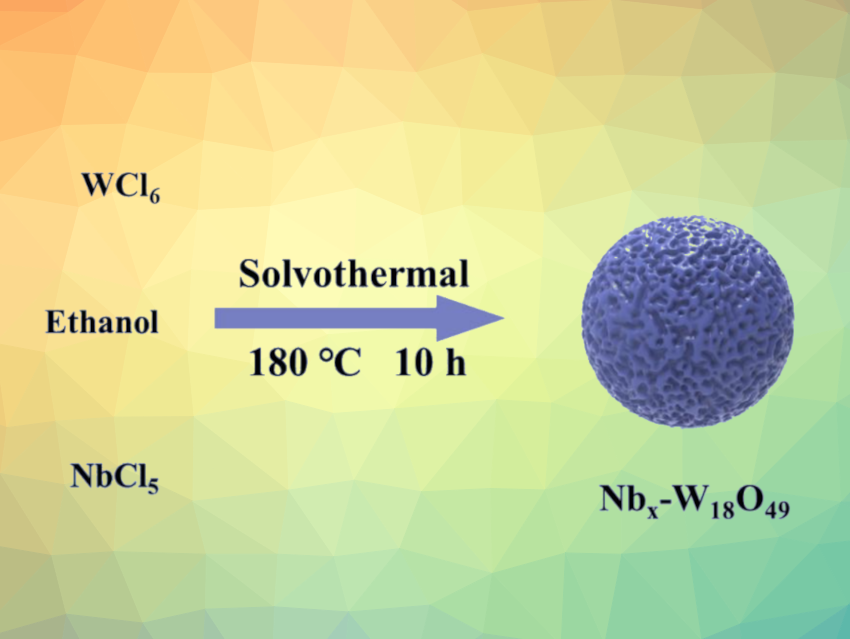
Mengyou Gao, Jianjian Lin, Qingdao University of Science and Technology, China, and colleagues have prepared niobium-doped tungsten oxide nanospheres that can serve as effective electrocatalysts for the HER. The team used a simple, one-pot solvothermal method under oxygen-free conditions for the synthesis of a series of Nbx-W18O49 electrocatalysts with varying Nb content. They mixed solutions of tungsten hexachloride (WCl6) and niobium pentachloride (NbCl5) in ethanol and heated the reaction mixture to 180 °C for 10{nbsp]h. The resulting products were washed, centrifuged, and freeze-dried to obtain the desired nanospheres.
The team found that the prepared Nb0.6-W18O49 electrocatalyst, in particular, showed a high hydrogen evolution activity in acidic environments and increased conductivity. The catalyst also showed good stability under different conditions. The niobium doping tunes the electronic structure of the tungsten oxide to improve its catalytic effectiveness. The doping increases the number of oxygen vacancies in the material, which, according to the researchers, work in synergy with neighboring tungsten active sites to accelerate the HER.
- Electronic Structure Modulating of W18O49 Nanospheres by Niobium Doping for Efficient Hydrogen Evolution Reaction,
Hui Guo, Lu Pan, Mengyou Gao, Linghui Kong, Jingpeng Zhang, Aslam Khan, Nasir A. Siddiqui, Jianjian Lin,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202403043