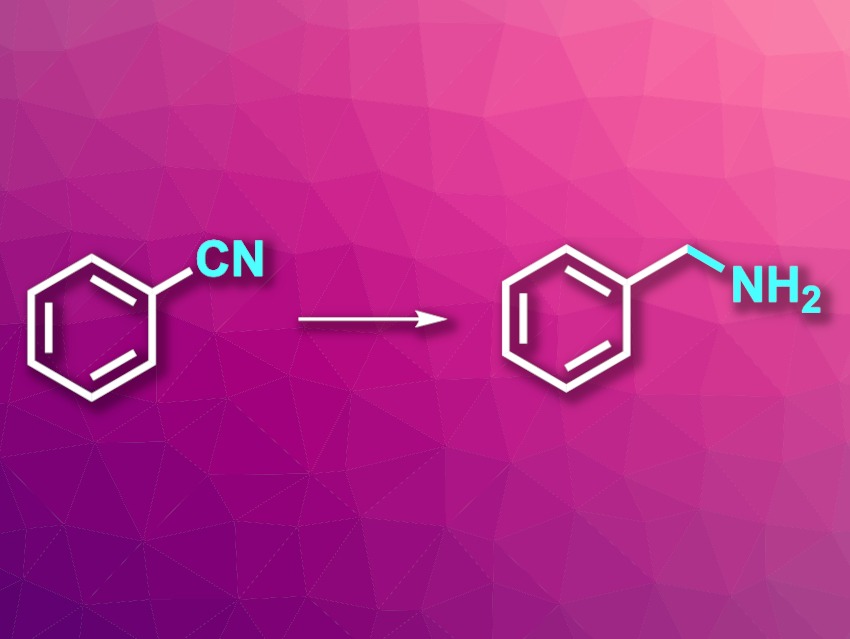
Hydrogenation reactions have a range of uses in organic synthesis. Catalytic hydrogenations can, for example, be used to prepare primary amines from nitriles (example reaction pictured) with high atom economy. However, existing catalysts for this type of reaction often suffer from a limited substrate scope with regard to structurally complex, functionalized nitrile substrates. Improved catalysts for more general hydrogenations would, thus, be useful.
Matthias Beller, Rajenahally V. Jagadeesh, Leibniz-Institut für Katalyse e.V., Rostock, Germany, and colleagues have developed a silica-supported nickel nanoparticle catalyst for the general and selective hydrogenation of a variety of nitriles. The team prepared the catalyst from Ni(NO3)2·6H2O, which was mixed with colloidal silica in water and heated to 65 °C. The material was then dried and calcined under air at 600 °C, giving nickel-oxide nanoparticles (NiO-NPs@SiO2). The nickel oxide was then reduced using H2 at 500 °C to obtain the active catalyst (Ni-NPs@SiO2-500).
The prepared catalyst was used for the hydrogenation of a wide range of heterocyclic, aromatic, and aliphatic nitriles under 35 bar H2 and 5 bar NH3 in methanol at 60–80 °C. The desired amines were obtained selectively in high yields, even for some of the more challenging substrates. The catalyst is stable and can be reused for several reactions.
- Stable and Reusable Ni-based Nanoparticles for General and Selective Hydrogenation of Nitriles to Amines,
Zhuang Ma, Matthias Beller, Stephan Bartling, Nils Rockstroh, Jagadeesh Rajenahally, Bei Zhou, Vishwas Chandrashekhar, Asma A. M. Alenad,
Chem. Sci. 2022.
https://doi.org/10.1039/d2sc02961h