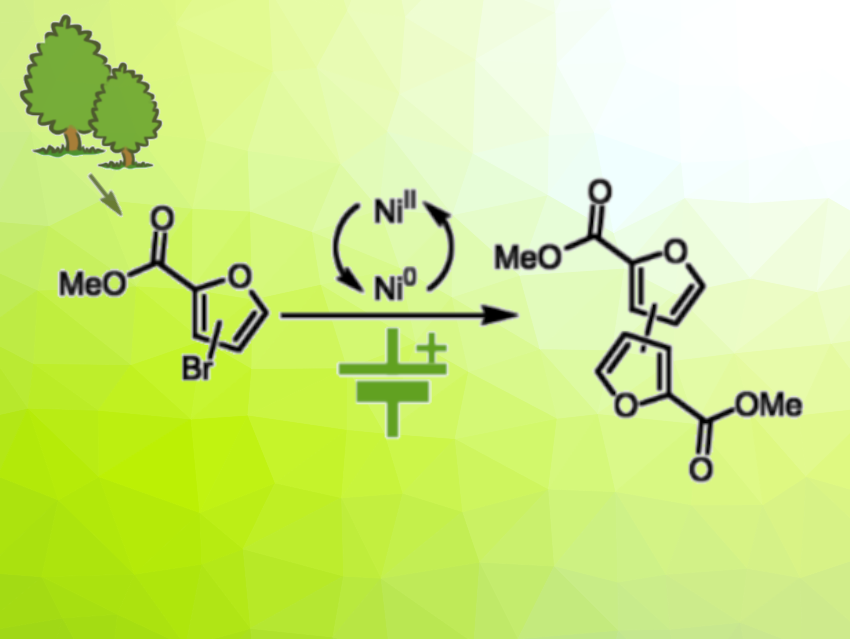
Polymers based on monomers prepared from renewable resources could be useful, environmentally friendly alternatives to fossil-resource-based materials. Furan-based materials such as polyethylene furan-2,5-dicarboxylate (PEF), for example, could be used to replace polyethylene terephthalate (PET). The monomer of PEF, 2,5-furandicarboxylic acid (FDCA), is usually prepared from lignocellulosic biomass. As an alternative, furan-based monomers can be prepared from hemicellulose. Bifuran polymers or bifuran-furan co-polymers have complementary properties to PEF, but the synthesis of bifuran monomers is less well-studied.
Tom Wirtanen, VTT Technical Research Centre of Finland Ltd., Espoo, Finland, and colleagues have developed a method for the nickel-electrocatalyzed synthesis of bifuran-based monomers (example pictured). The monomers are prepared from methyl bromo-furancarboxylates, which, in turn, can be synthesized from hemicellulose-based furfural. The team dissolved different bromo-functionalized furans, thiophenes, or arenes in dimethylformamide (DMF) together with Ni(dmbpy)Br2 (dmbpy = 4,4′-dimethyl-2,2′-bipyridine) as a catalyst, tetrabutylammonium tetrafluoroborate (TBABF4) as an electrolyte, and triethanolamine (TEOA) and subjected the solution to electrolysis in an undivided cell at 50 °C with graphite electrodes. The products were isolated by precipitation.
The team obtained the desired bifuran-based monomers in yields of up to 82 %. In particular, the method was used to prepare 2,2′-bifuran-5,5′-dicarboxylic acid dimethyl ester and 3,3′-bifuran-5,5′-dicarboxylic acid dimethyl ester, which are key constituents in bio-based bifuran polymers and bifuran-furan co-polymers. The method does not require sacrificial anodes due to the use of triethanolamine as an electron donor.
- Nickel‐Electrocatalyzed Synthesis of Bifuran‐Based Monomers,
Valtteri Oksanen, Sari Rautiainen, Tom Wirtanen,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202302572