Many biologically active molecules contain heterocyclic aromatic groups. Thus, efficient routes for enantioselective syntheses of functionalized heteroaromatics are important. One approach for this is the direct activation of C–H bonds, avoiding the synthesis of intermediates with reactive functional groups. Selective C–H functionalizations by late transition metals, for example, are known, but these metals are often rare and expensive. Nickel is more abundant and, therefore, cheaper and can exhibit similar catalytic activity in some reactions.
John Montgomery and Mo Chen, University of Michigan, Ann Arbor, USA, have developed an enantioselective C–H alkylation of heterocyclic aromatics using a nickel catalyst with an N-heterocyclic carbene (NHC) ligand, stabilized by an additional diene ligand. The reactions were performed in toluene at room temperature with potassium bis(trimethylsilyl)amide (KHMDS) as a base and 5 mol% of the catalyst, formed in situ from the corresponding NHC precursor and Ni(COD)2.
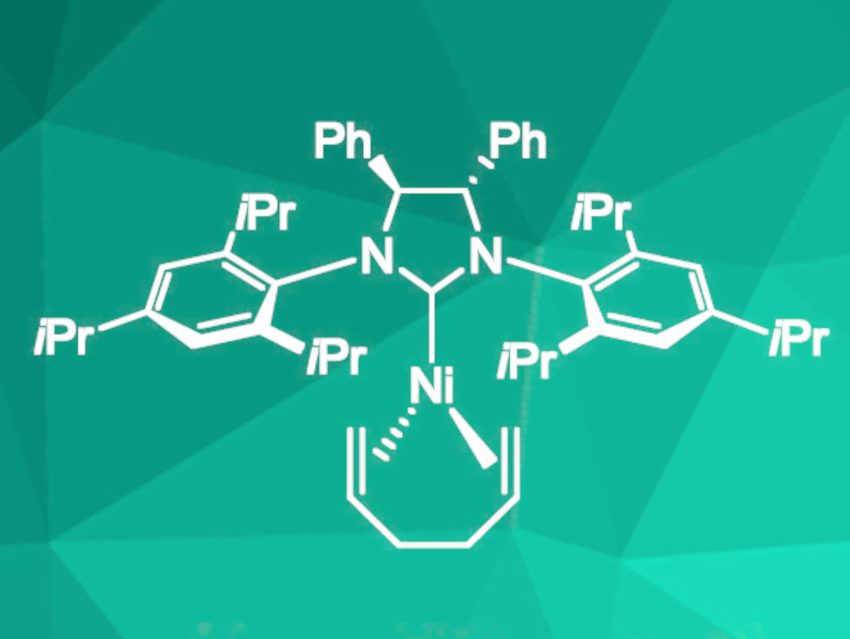
This procedure was efficient for electron-rich benzoxazole derivatives and norbornene as substrates, but not for benzoxazole derivatives with electron-withdrawing groups. Benzofurans and benzimidazoles were also successfully converted, but required elevated temperatures of 60 °C. To extend the scope of the reaction to electron poor-substrates, a nickel catalyst with 1,5-hexadiene instead of cyclooctadiene (pictured) was used. This led to the desired improvement in scope and decreased the required catalyst loading to 2.5 mol%.
Preliminary tests with styrene instead of norbornene were also carried out. This reaction led to the desired products, but the regioselectivity and enantioselectivity still need improvement. The researchers propose a reaction mechanism that involves a ligand-to-ligand hydrogen transfer. The developed synthesis route provides easy access to a wide range of alkylated heteroaryl substrates.
- Nickel-Catalyzed Intermolecular Enantioselective Heteroaromatic C–H Alkylation,
Mo Chen, John Montgomery,
ACS Catal. 2022.
https://doi.org/10.1021/acscatal.2c03228