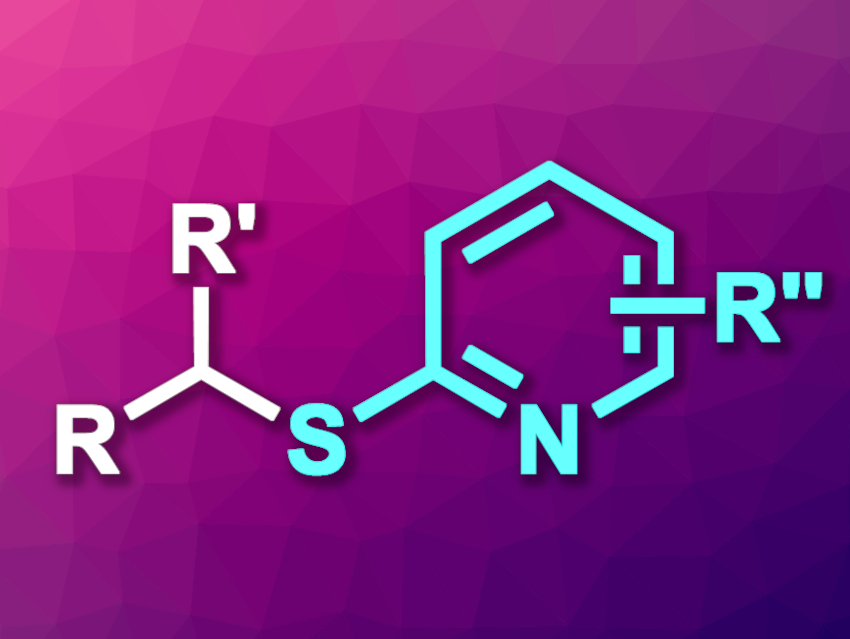
Sulfides are organic compounds that have applications in many fields, ranging from drug development to materials chemistry. Alkyl pyridyl sulfides, for example, are found in drugs such as antibiotics or antihypertensives (blood pressure medications). New methods for the synthesis of this type of compound are, thus, interesting research targets.
Xinxin Shao, Yang Ye, Hangzhou Normal University, China, and colleagues have developed a method for the nickel-catalyzed electrochemical synthesis of alkyl pyridyl sulfides (general product structure pictured) from readily available alkyl alcohols and pyridyl thioesters. The team used nickel(II) triflate as the catalyst together with 2,2′-bipyridine-4,4′-dicarboxylic acid (dcbpy) as a ligand, PPh3, tetrabutylammonium bromide (TBAB) as an electrolyte, and 4-dimethylaminopyridine (DMAP) as a base. The reactions were performed at room temperature in an undivided electrochemical cell with a nickel cathode and a carbon anode, using N-methyl-2-pyrrolidone (NMP) as the solvent.
Under these conditions, the team reacted a variety of alkyl alcohols with pyridyl thioesters. The desired alkyl pyridyl sulfides were obtained in moderate to high yields. The method has a broad substrate scope and tolerates a range of fucntional groups. The researchers successfully employed it for the synthesis of natural products and pharmacautically active compounds.
- Nickel-Catalyzed Electroreductive Sulfuration of Alkyl Alcohols with Pyridyl Thioesters,
Lingling Deng, Ying Lin, Xinxin Shao, Yang Ye,
Org. Lett. 2025.
https://doi.org/10.1021/acs.orglett.4c04665