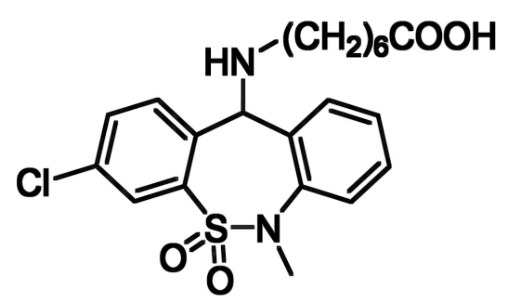
Tianeptine (pictured below) is an antidepressant drug, which acts as a μ-opioid receptor full agonist. Derivatives of this biologically active compound with different substituents at the nitrogen atom in the central ring, including fused rings and bridging structures, have been synthesized. N-demethylation is important in the metabolism of tianeptine. Thus, these analogues could show different pharmacological properties from the parent compound.
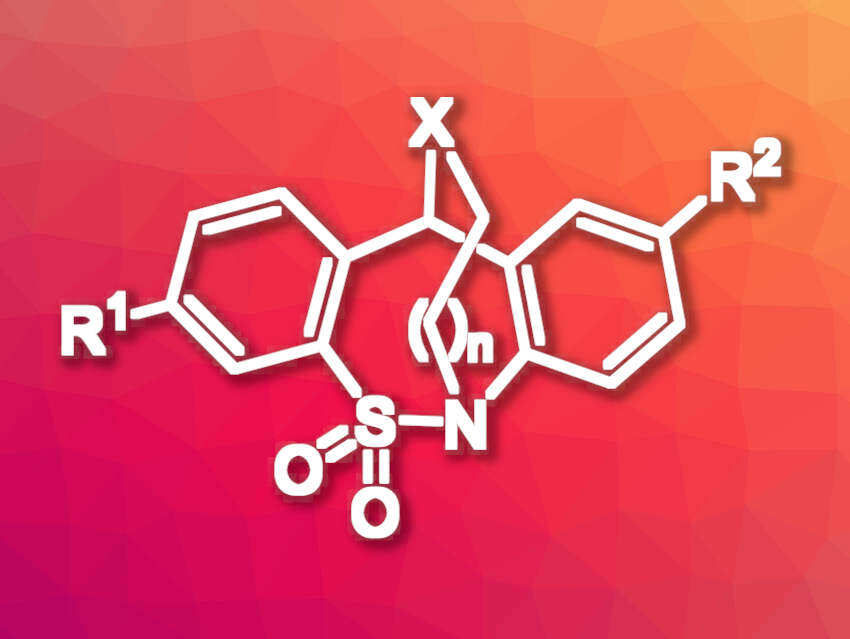
Balázs Volk, Egis Pharmaceuticals Plc., Budapest, Hungary, and colleagues have synthesized five new, bridged, tianeptine–like tetracyclic ring systems featuring a dimethylene or trimethylene linker between the nitrogen atom of the thiazepine ring and the nitrogen, oxygen, or sulfur atom linked to the C(11) carbon (general structure pictured, X = O, S, NR). The team first prepared a common intermediate with a carbonyl group in the seven-membered ring, which was then used to connect the desired bridging substructures. They used 2-bromoethanol or 3-bromopropan-1-ol to introduce two- or three-carbon bridges, respectively.
Heterocyclic ring systems are useful in drug discovery—many new small-molecule drugs have a heterocyclic core structure. Thus, the new tetracyclic heterocyclic compounds developed in this work could be interesting for further studies in the field of medicinal chemistry.
- Synthesis of New Tetracyclic Ring Systems: Bridged Derivatives of Hydroxy‐, Sulfanyl‐ and Amino‐Substituted Dibenzo[c,f][1,2]Thiazepine S,S‐Dioxides,
Gábor Berecz, Dávid Szabó, András Dancsó, Mária Tóthné Lauritz, Loránd Kiss, Gyula Simig, Balázs Volk,
Eur. J. Org. Chem. 2024.
https://doi.org/10.1002/ejoc.202400835