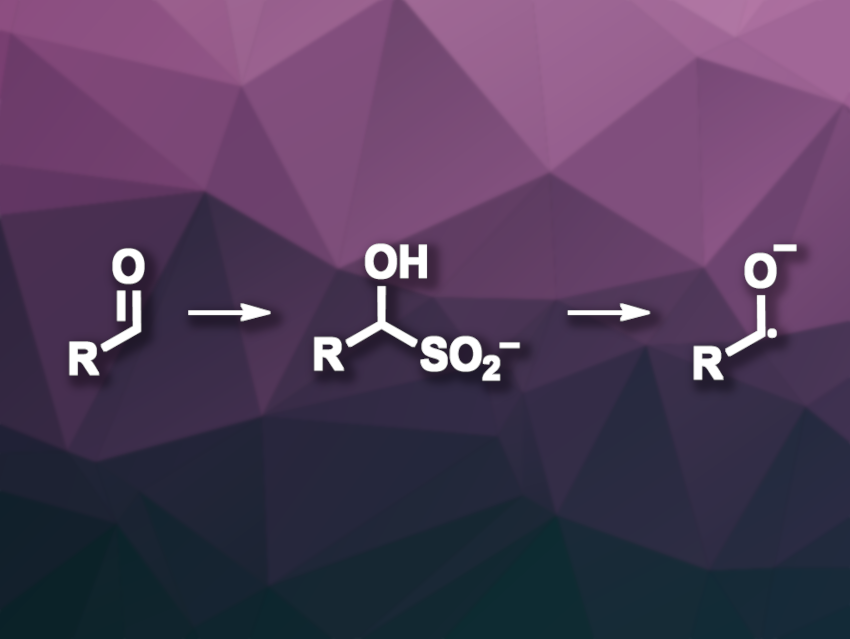
Ketyl radicals can be useful intermediates in organic synthesis, extending the traditional reactivity of carbonyl compounds as electrophiles. However, accessing them directly via a single-electron reduction of the corresponding carbonyl compounds can be challenging due to the reduction potentials of the precursors. The necessary highly reducing conditions can limit the functional group tolerance of transformations.
Adam Noble, University of Bristol, UK, and colleagues have developed an alternative approach to the generation of ketyl radical species and used it for an aldehyde-alkene coupling. The team employed α-hydroxy sulfinates (pictured) as intermediates in the radical generation. They reacted a range of different aldehydes with sodium sulfoxylate (Na2SO2), which was generated in situ via the hydrolysis of thiourea dioxide (TDO) in the presence of NaOH. This was followed by irradiation with blue LED light in the presence of eosin Y as a photocatalyst, which allowed the formation of ketyl radical anions via oxidative desulfination and the subsequent coupling with a range of alkenes.
The desired coupling products were obtained in mostly moderate to high yields. The reaction can also be used for intramolecular couplings to achieve ring formations in aldehyde-functionalized olefins. Overall, the work provides a method for the generation of ketyl radical anions without a need for strongly reducing conditions.
- Aldehyde–Olefin Couplings Via Sulfoxylate-Mediated Oxidative Generation of Ketyl Radical Anions,
Zhihang Li, Joseph A. Tate, Adam Noble,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c10093