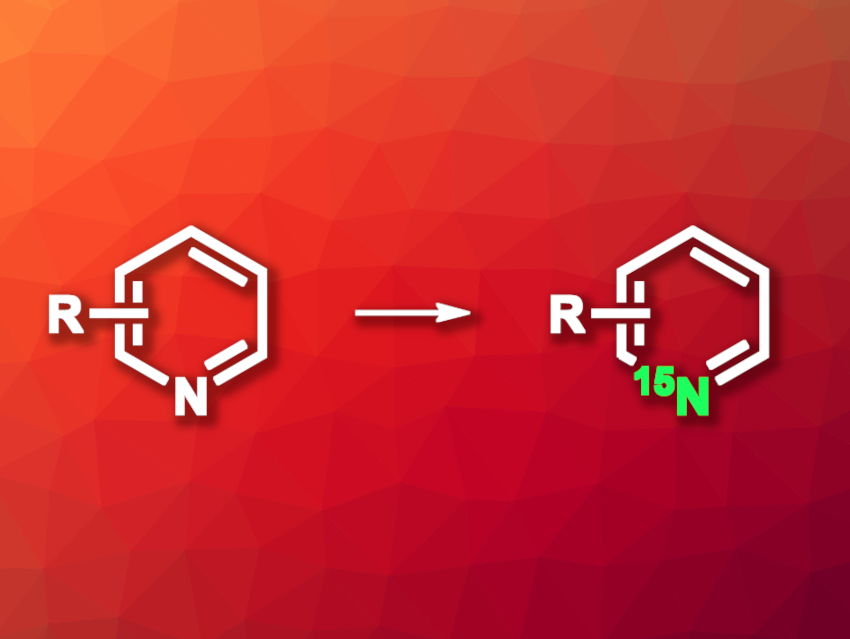
Nitrogen-containing heterocycles are important structural motifs in organic chemistry and are often found in biologically active compounds. Labeling this type of compound with radioactive isotopes can be useful in biological and medicinal chemistry research. There are approaches to deuterium and 13C labeling, but they can have drawbacks such as limited site-selectivity or a need for the synthesis of the compounds from simple 13C feedstocks. Labeling with 15N can also be challenging due to a need for synthesis from ammonia or ammonium salts, which limits the use of this label.
Joel M. Smith, Florida State University, Tallahassee, USA, and colleagues have developed a method for the synthesis of 15N-labeled azines (six-membered heteroarenes containing nitrogen atoms) in which a nitrogen atom is exchanged for a 15N atom using a reagent specifically designed for the reaction. The team’s approach is based on ANRORC (Addition of Nucleophile, Ring Opening, Ring Closing) reactions, in which the aromatic ring is opened and then closed again to allow for the exchange. They first activated different azines using a 2,4-dinitro-1-tosylbenzene to give the corresponding pyridinium salts. The activated intermediates were then reacted with 15N-2,4,6-trimethoxybenzylamine as a nucleophile under microwave irradiation, which gave the desired 15N-labeled azines via an ANRORC-type mechanism.
The desired products were obtained in yields of 33–92 % and with high 15N enrichment. The team successfully labeled a variety of pyridines, isoquinolines, azaindoles, and benzoisoquinolines, for example. Azines with multiple nitrogen atoms or multiple electron-withdrawing groups were not suitable substrates because they did not undergo the required activation. The researchers used the developed approach for the formal synthesis of the 15N-labeled drug solifenacin, demonstrating its potential for use in pharmaceutical chemistry.
- 15NRORC: An Azine Labeling Protocol,
Zachary A. Tolchin, Joel M. Smith,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c11618