Protein S-palmitoylation—the reversible attachment of the fatty acid palmitate to specific cysteine residues—plays a central role in regulating where proteins go in a cell, how stable they are, and how they interact. This lipid modification is catalyzed by a family of enzymes known as palmitoyl-S-acyltransferases (PATs or ZDHHC enzymes), which share a conserved four-amino acid active-site motif: aspartate-histidine-histidine-cysteine (DHHC). These enzymes typically localize to internal membranes such as the Golgi apparatus and endoplasmic reticulum (ER), though some are found at the plasma membrane.
The dynamic interplay between palmitoylation and depalmitoylation, catalyzed by enzymes such as APT1, APT2, and PPT1, occurs on timescales ranging from seconds to hours. Disruptions in this balance are implicated in a wide range of diseases, including cancer, where proteins like HRas, EGFR, and ERK1/2 rely on this lipid tagging for proper function. Conventional inhibitors used in such cases like 2-bromopalmitate (2-BP) are highly toxic and interfere with unrelated enzymes in lipid metabolism and protein diacylation.
To develop biochemical tools for studying and potentially correcting abnormal protein palmitoylation, Ines Neundorf, University of Cologne, Germany, and colleagues have synthesized peptides containing the DHHC motif and fused them to a cell-penetrating sequence (sC18*). The idea was that these DHHC-containing peptides could enter cells and interact with the palmitoylation machinery or substrates, thereby modulating palmitoylation dynamics.
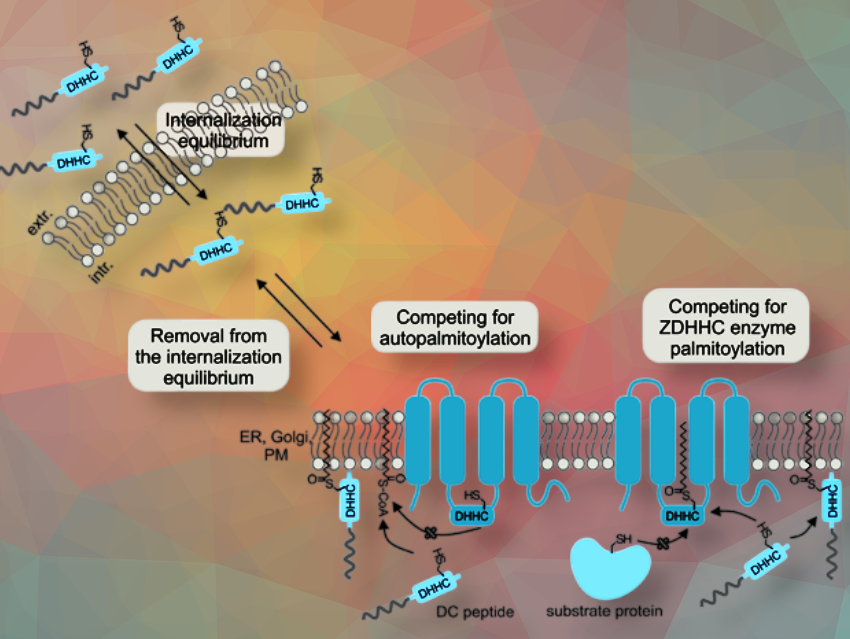
The researchers generated a library of so-called DC peptides and screened them for cellular uptake and cell compatibility. After an initial screening for cellular uptake and cytotoxicity, the team analyzed the intracellular fate and activity of the most interesting candidate, peptide DC-2, in more detail. They found that DC-2 was directly removed from an internalization equilibrium and further processed via enzymes.
In cancer cell lines, including HeLa (cervical cancer) and SW480 (colon cancer) cells, DC-2 appeared to alter the cellular distribution and activity of key palmitoylated proteins such as HRas and ERK1/2. It also enhanced the responsiveness of the epidermal growth factor receptor (EGFR) to its ligand, suggesting that the peptide affects palmitoylation-dependent signaling events at the membrane.
The researchers propose that their approach offers a targeted biochemical strategy to interfere with protein lipidation in living cells. Unlike broad-spectrum inhibitors, these motif-based peptides may allow for more selective modulation of disease-relevant protein modifications with fewer off-target effects.
- First steps towards the design of peptides that influence the intracellular palmitoylation machinery,
Ines Neundorf, Katharina Stillger, Eric Platz-Baudin, Florian Friedland, Melina Ruppel, Coco-Louisa Sticker, Anne Bodenhausen, Eric Noetzel
ChemBioChem 2025.
https://doi.org/10.1002/cbic.202500218