Late-stage methylation is a useful type of reaction in drug development. The so-called “magic methyl effect” refers to the improved biological activity, selectivity, or stability of molecules that can result from adding a methyl group. New methods for the regioselective introduction of methyl groups, e.g., at arenes are interesting research targets.
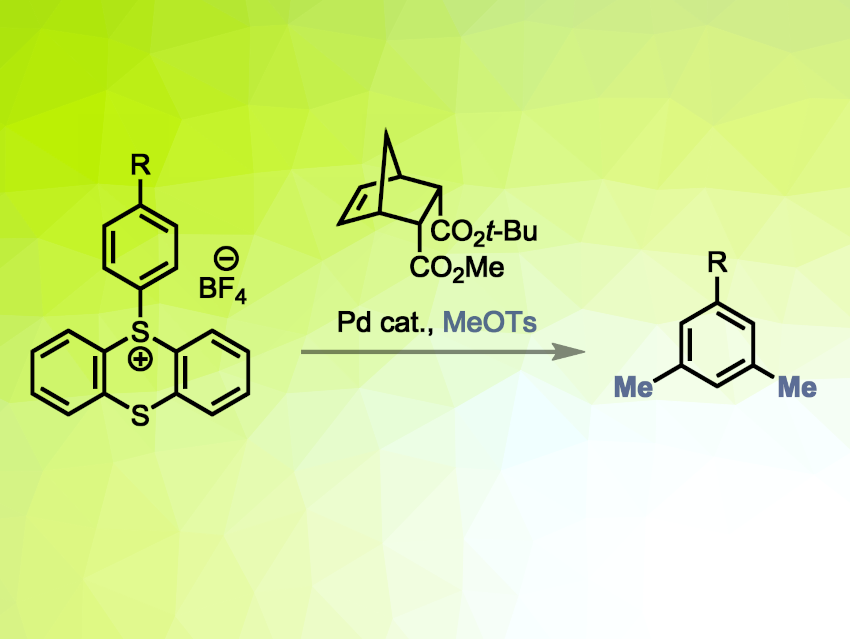
Tobias Ritter, Max Planck Institute for Coal Research, Mülheim an der Ruhr, Germany, and colleagues have developed a reaction of aryl thianthrenium salts that allows the selective dimethylation of the meta positions of arenes. The team combined a site-selective thianthrenation with a Catellani reaction to access 3,5-dimethylated arenes (general reaction pictured). The team first prepared thianthrenium salts obtained from mono-substituted arenes via reactions with thianthrene S-oxide. This was followed by the dimethylation step, using methyl tosylate, a palladium-based catalyst, sodium formate as a hydride source, tetramethylammonium iodide (TMAI) as an additive, and a norbornene as a “traceless” directing group.
Using this approach, a variety of meta-dimethylated products were obtained in mostly moderate yields. Complex, drug-like molecules were suitable substrates. The developed reaction is complementary to the existing ipso-alkylation of aryl thianthrenium salts and extends the options for late-stage methylations of arenes via aryl thianthrenium salts.
- Meta‐Dimethylation of Arenes via Catellani Reaction from Aryl Thianthrenium Salts,
Michał Mrozowicz, Sagnik Chatterjee, Markella Aliki Mermigki, Dimitrios Pantazis, Tobias Ritter,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202419472



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)