Aryl chlorides can be considered preferred reagents in palladium-catalyzed arylation reactions due to their affordability. However, the reactivity of aryl chlorides is often lower than that of aryl bromides and iodides due to significantly higher barriers for oxidative addition. Thus, the use of aryl chlorides can require specially designed ligands for Pd catalysts.
Victor M. Chernyshev, Platov South-Russian State Polytechnic University (NPI), Novocherkassk, Russia, Valentine P. Ananikov, Platov South-Russian State Polytechnic University (NPI) and Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Moscow, and colleagues have developed a new type of N-heterocyclic carbene (NHC) ligand (example pictured). The team’s design provides enhanced electron donation from the NHC to the Pd center and facilitates the oxidative addition of aryl chlorides, significantly improving catalytic efficiency.
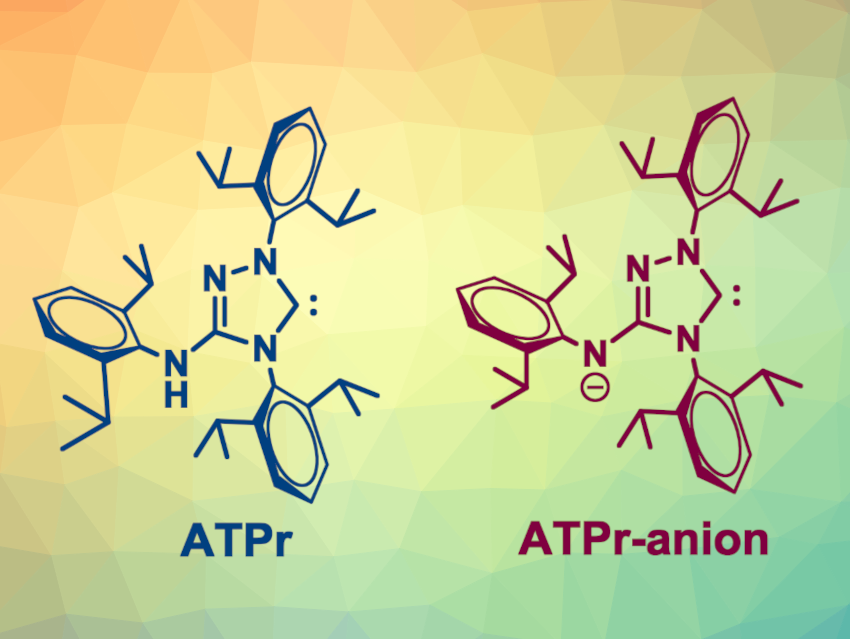
The researchers combined specific steric properties and an anionic nature in NHC ligands based on 1,2,4-triazol-5-ylidenes. The steric requirements are fulfilled by ortho-disubstituted N-aryl groups adjacent to the carbene center. In addition, the ligands can be ionized under basic conditions due to their NH-acidic aryl(alkyl)amino groups.
An optimized ligand, named ATPr (pictured), features 2,6-diisopropylphenyl groups. When used in palladium-catalyzed reactions, the ligand led to improved catalytic efficacies—in particular, in Suzuki-Miyaura, ketone α-arylation, and Buchwald-Hartwig reactions of unactivated aryl chlorides. According to the team, the work could provide useful insights for further advancements in the design of NHC ligands for catalysis.
- Base‐Ionizable Anionic NHC Ligands in Pd‐catalyzed Reactions of Aryl Chlorides,
Andrey Chernenko, Victoria Baydikova, Vadim Kutyrev, Alexander Astakhov, Mikhail Minyaev, Victor Chernyshev, Valentine P. Ananikov,
ChemCatChem 2023.
https://doi.org/10.1002/cctc.202301471