The controlled, bottom-up synthesis of carbon nanotubes (CNTs) is an interesting research target because it could allow the preparation of CNTs with defined lengths and diameters to tailor them for different applications. Such an approach can, for example, start from [n]cycloparaphenylenes ([n]CPPs), which can be considered segments of an armchair CNT and can be elongated to form nanotubes. Functionalized [n]cycloparaphenylene acetylenes ([n]CPPAs), which are composed of alternating phenylene and acetylene units, could also serve as precursors. The alkyne groups can be transformed to, e.g., naphthalenes (pictured in cyan below) via benzannulation reactions. In theory, a Scholl oxidation step could then be used to fully fuse the structure and form a carbon nanotube.
Marcel Mayor, University of Basel, Switzerland, Karlsruhe Institute of Technology (KIT), Germany, and Sun Yat-Sen University (SYSU), Guangzhou, China, and colleagues have tested this approach experimentally. They first prepared a [12]CPPA (pictured), which, according to the team, is the largest reported [n]CPPA so far. They started from 1,4-diiodo-2,5-dibromobenzene, which was coupled with [(3-cyanopropyl)di-isopropylsilyl]acetylene (CPDIPSA). Two equivalents of the resulting intermediate were connected using a dialkyne-type linker, functionalized with methoxynaphthalene units, trimerized using 1,4-dibromobenzene linkers, and subjected to a final aromatization step to give the desired [12]CPPA.
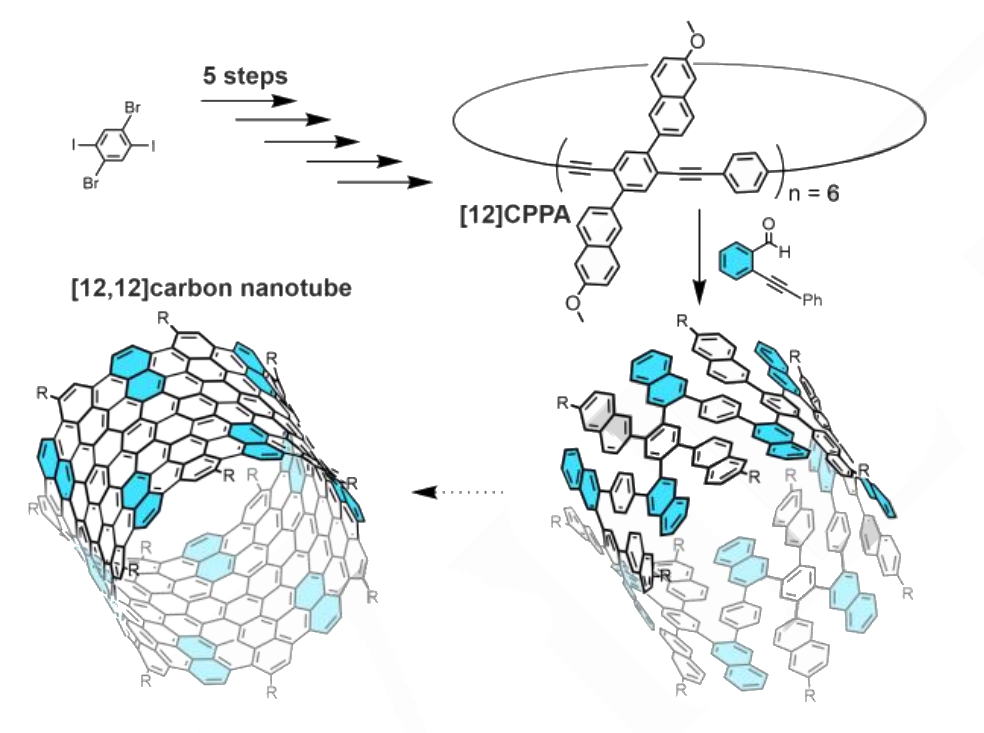
The [12]CPPA shows bright turquoise fluorescence with a high quantum yield of 77 %. It successfully underwent a twelve-fold benzannulation to give a carbon nanotube precursor (pictured above in the bottom right corner). The final step in the carbon nanotube synthesis, a Scholl oxidation turned out to be challenging, however. The team hopes to improve the reaction sequence to the precursor and obtain higher quantities for reaction optimization of the Scholl oxidation. Nevertheless, the method could already be useful for the preparation of various conjugated macrocycles.
- A Cycloparaphenylene Acetylene as Potential Precursor for an Armchair Carbon Nanotube,
Eric Sidler, Ramon Röthlisberger, Marcel Mayor,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202403084
![New Largest [n]Cycloparaphenylene Acetylene](https://www.chemistryviews.org/wp-content/uploads/2024/09/12cpps2024.png)